Build Your
Document Repository
with Flexibility
Create & Edit
Create, edit, and format SOPs, manuals, and protocols with advanced tools for professional formatting, structured templates, and automatic pagination.
Upload
Upload documents and store in a digital document repository through bulk import, drag-and-drop, email-based upload, or monitored folders with metadata tagging.

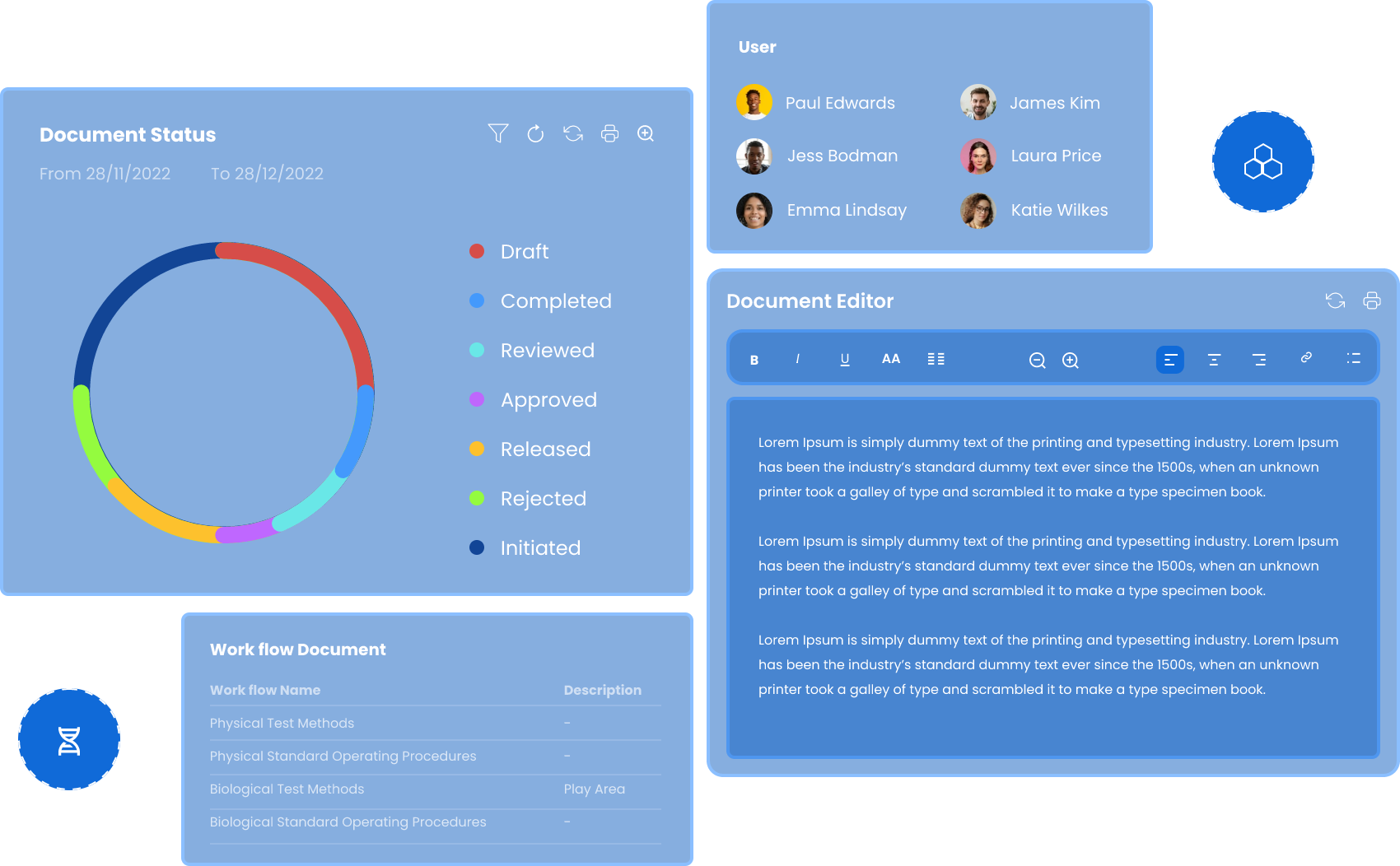
Your Central Hub for Total Document Control
Transform how your team works with documents with Qualis DMS. Move beyond scattered files and chaotic folders to a secure and intelligent platform where every document has its place and purpose
Create, store, track, and manage documents throughout their entire lifecycle, from initial draft to final approval and distribution effortlessly.
Single Source of Truth
Keep all your files organized and accessible in one secure place.

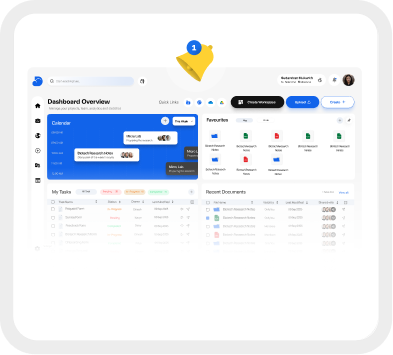
Intuitive Dashboard & Alerts
Get a comprehensive overview of what's happening with your team.
Access Control
Define user roles & permissions to maintain data security.

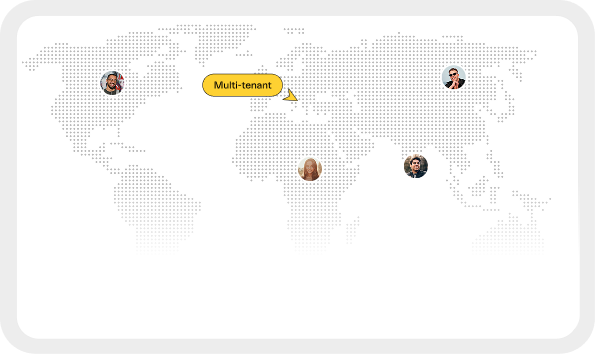
Multi-Tenant & Multi-Site
Supports multi-tenant & multi-site architecture, allowing segregated control
Secure Sharing
Share documents safely using access control.


Single Source of Truth
Keep all your files organized and accessible in one secure place.

Intuitive Dashboard & Alerts
Get a comprehensive overview of what's happening with your team.

Access Control
Define user roles & permissions to maintain data security.

Multi-Tenant & Multi-Site
Supports multi-tenant & multi-site architecture, allowing segregated control

Secure Sharing
Share documents safely using access control.
Hassle-Free Solution for All Your Document Management Needs

Advanced Document Authoring
Structured authoring tools for professional document creation

Template & Form Management
Create reusable templates enabling consistent structured data entry

Document Lifecycle Management
Document lifecycle control with complete auditability

Workflow Automation Engine
Review and approve documents within defined workflow structures

Version & Release Control
Automated version tracking with controlled document releases
Structured Workspace with Instant Retrieval
Build a document structure that makes perfect sense for your business
-
Workspaces & Nested Folders
Classify everything by department, client, or project in an easy-to-navigate interface.
-
Custom Tags & Metadata
Smart full-text and metadata search allows users to locate documents based on tags, content, version, author, or audit events, across multi-site repositories.


Standardized Templates & Seamless Document Transfer
Build a document structure that makes perfect sense for your business
-
Automate with Smart Templates
Design fillable templates and forms with structured fields. Standardize data capture, reduce manual entry, and accelerate your workflows
-
Multi-Format File Support
Natively handle Microsoft Office, PDF, Images, AutoCAD, Rich Text, and more
-
Effortless Bulk Uploads
Migrate entire projects or archives in a single step with our secure bulk upload feature

Version & Print Control
Qualis DMS enables quick release and printing of documents to hard copies
-
Version Control
Automatic versioning with comprehensive audit trails enables traceability of all document changes
-
On-Demand Publishing
Print or publish any selection of documents on-demand
-
Version-Controlled Publishing
Ensure your output is generated from the correct file version
-
Controlled Print Management
Setup controlled access & approvals for printing and reprinting activities
Typical Document Workflow in Qualis DMS

Automate Form Filling
Designed to align with FDA/MHRA cGMP guidelines.
-
Auto-Populate Details
Autofill form headers with dynamic values: batch number, product name, issuance date & time.
-
Launch Seamless Workflows
Auto-trigger workflows upon form submission for use cases like calibration, deviations, or incident reporting.


Workflow Management
Set role-based privileges for document review and e-sign approval. Qualis DMS’s engine supports serial, parallel, and hybrid workflows, tailored for each document type
-
Business Process Management (BPM)
Define templates for complex document-driven workflows such as CAPA, deviation handling, or audit responses. Workflow templates support cascade escalations and multi-stage reviews.
-
Case Management
Group related documents into a centralized case file (e.g. CAPAs, audits, lab reports) with milestone tracking, linked-file associations, and condition-based branching workflows.

Secure Your Digital Assets & Stay Audit-Ready
Ensure your team always works with the correct information while protecting your most sensitive data
-
Password Policy Control
Configurable password rules and authentication mechanisms can be set as per preference
-
Comprehensive Audit History
Maintain a complete record of every action that takes place to a file throughout its lifecycle
-
Electronic Records & E-signature Support
Fully compliant with 21 CFR Part 11, EU Annex 11, and ISO standards
Make Your
Document Management Compliant
Ensure all documents are up-to-date, accurate, and easily accessible to authorized personnel

Automatic Version Control
Always have the latest, approved version at your fingertips. No more "Final_v2_final"

Ensure Traceability
Track changes and approvals with precise version & release control

Access Control Tiers
Tag documents by sensitivity (Confidential, Internal, etc.) to ensure only the right people see the right information
Cloud-Ready Integration

Sync with SharePoint, Google Drive, or OneDrive seamlessly

Auto-import documents from monitored folders

Publish documents to network folders or team libraries for collaboration

Sync with SharePoint, Google Drive, or OneDrive seamlessly

Auto-import documents from monitored folders

Publish documents to network folders or team libraries for collaboration
Benefits of Switching to Qualis DMS

Paperless System
Standardized browser-based access eliminates physical storage dependency.
Low Compliance Cost
Built-in role-based control and traceability reduce audit-related expenses

Improved Collaboration
Central access improves teamwork and document ownership visibility

Scalable & Extensible
Ideal for multi-site or multi-department deployment
Would you like to learn more about Qualis DMS?
Submit this form and our sales representative will contact you soon. E-Mail us: [email protected]