Driven by regulatory compliance and having to ship products across the globe, it becomes all the more complex for the pharmaceutical industry to comply with the regulations of various countries.
Going digital is no more a luxury but a de facto standard for the industry.
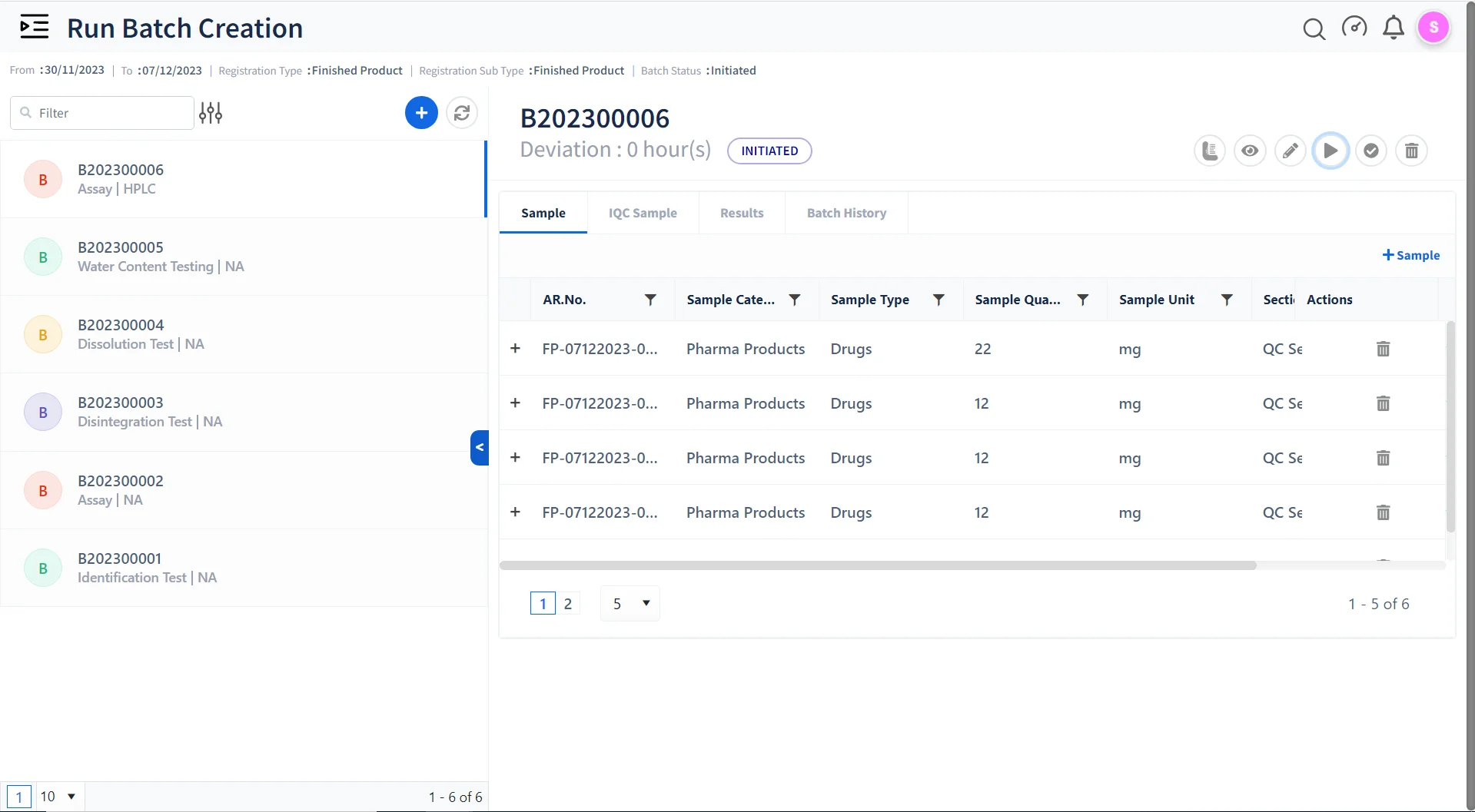
Ensuring Quality Control & Compliance with Qualis LIMS:
Batch Testing & Release
Monitor and manage batch-to-batch consistency in drug manufacturing to maintain the highest quality.
Stability management
Perform Stability studies on finished products in a paperless compliant environment.
Annual Product Quality Reporting
Easily generate detailed reports to meet pharmaceutical compliance and performance standards.
Environmental Monitoring
Create Study Plans for environmental monitoring, capture EMS data, and generate Reports.
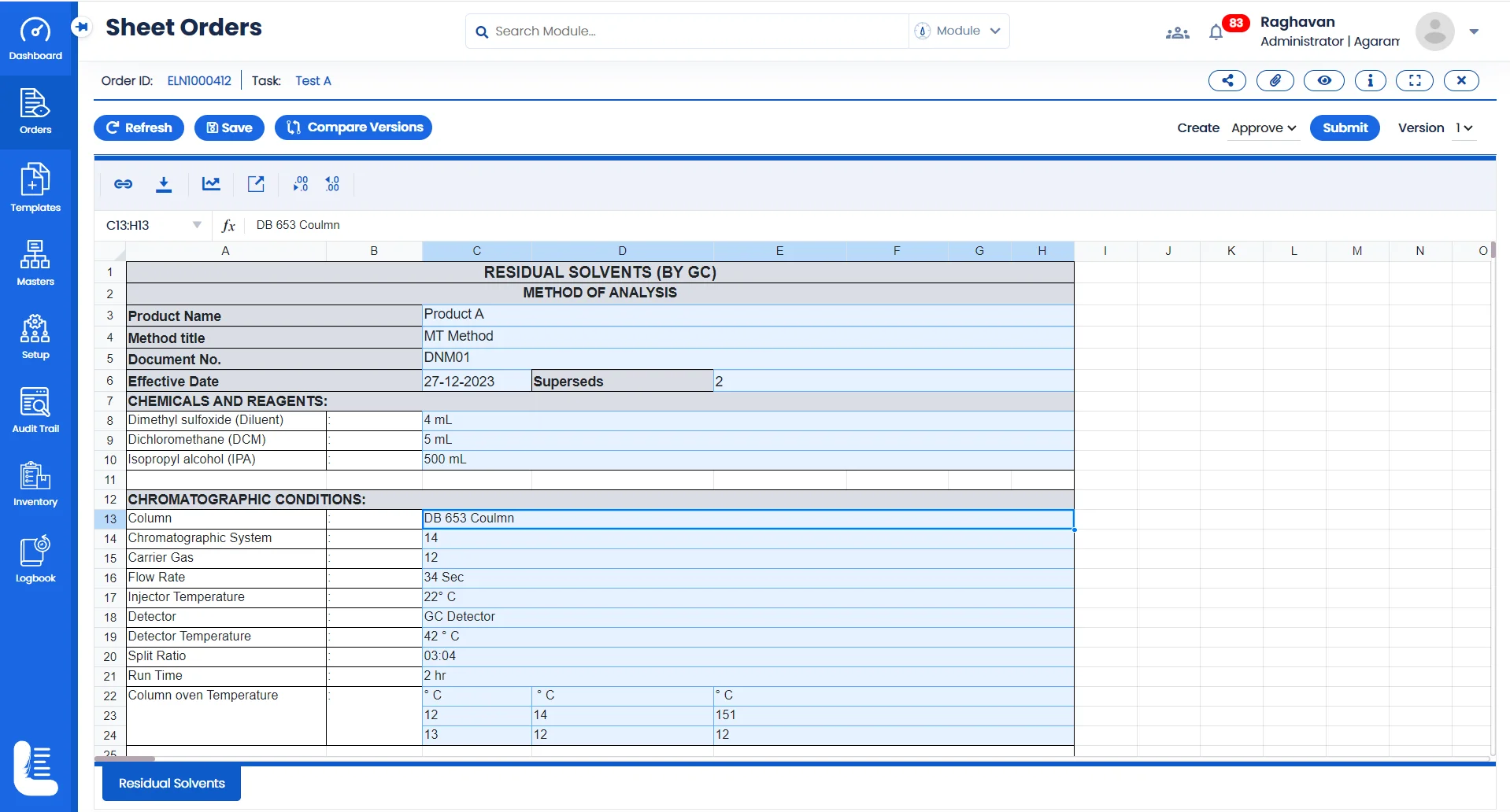
Electronic Method Execution using Logilab ELN
Test Method Recording & Execution
Make all your Test Data sheets electronic; Record results, and observations digitally. This digital recordkeeping ensures that critical findings are securely stored and easily accessible.
Data Traceability
Drug discovery often involves cross functional teams. Logilab ELN features a robust data version and release control capabilities to comply with regulatory compliance requirements.
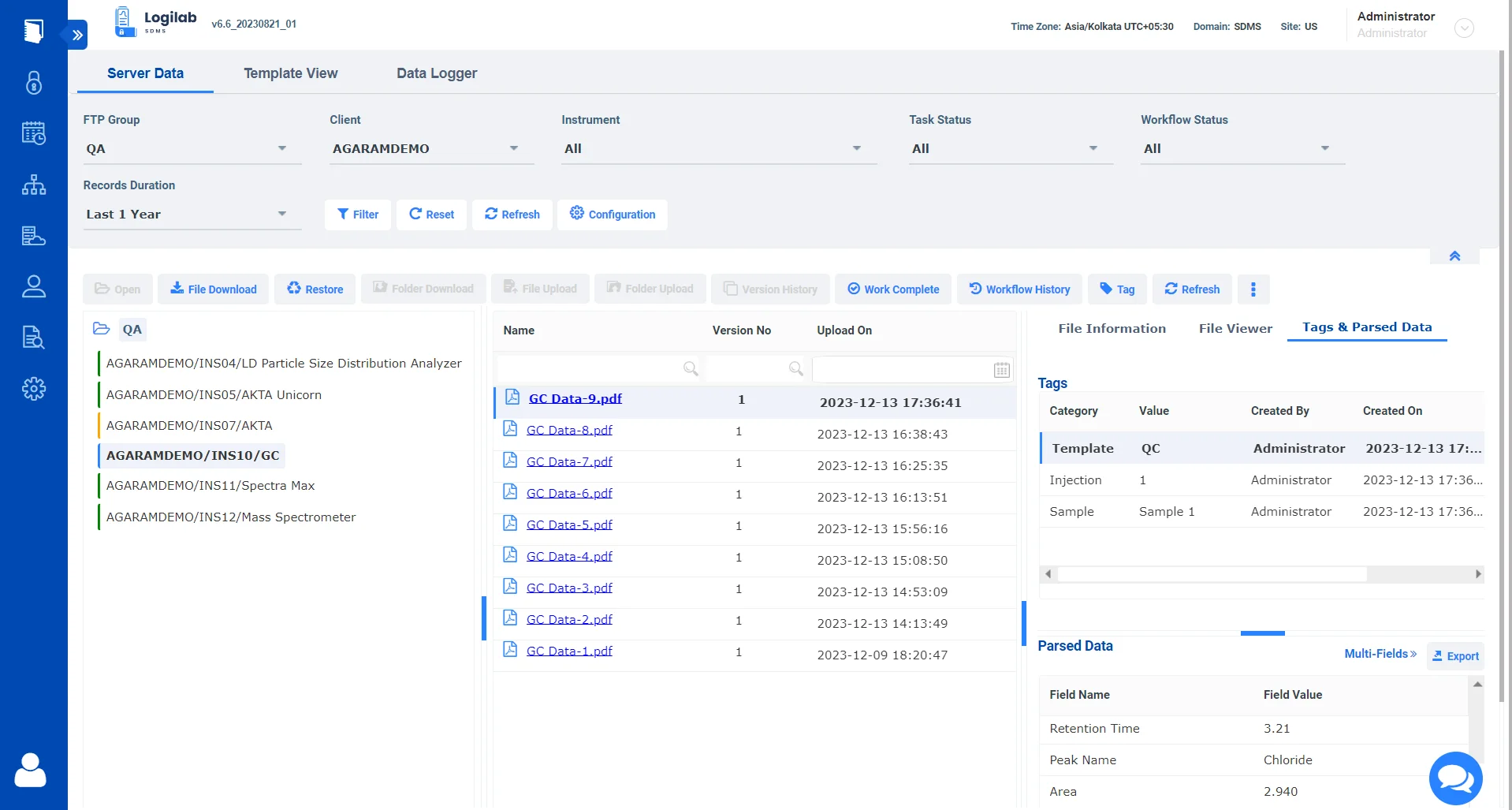
Optimizing Instrument data retrieval and management with Logilab SDMS
Instrument Integration
Seamlessly integrate with lab instruments for streamlined capture, catalog and archival of instrument raw data & metadata.
Data Integrity
Protect the integrity of your data by creating a single source of truth for all your instrument data.
Compliance Management
Comply with ALCOA++ Data integrity standards and comply with 21 CFR Part 11 regulatory compliance requirements.
Benefits of Our Solutions
Web-based solution
Reduce dependency on paper-based systems & physical storage. Our web based solution supports any modern browserand client OS

Reduce time and costs
Powerful searching & retrieval capability allows you to increase efficiency in terms of retrieving documents.

Secured storage and recovery
All document data & metadata are encrypted, stored on a central secure database, and are recoverable in case of a disaster. Keep your data protected!

Face audits with confidence
Allow your organization to stay in line with compliance requirements such as USFDA, Eudralex annex 11 & ISO17025.
Face Audits with Confidence: Data Integrity & 21 CFR Part 11 Compliance
Empower your pharmaceutical operations with Logilab ELN, Logilab SDMS, and Qualis LIMS. These solutions provide essential features to streamline workflows, enhance data precision, and ensure compliance with industry standards.
Achieve unparalleled data integrity in every aspect of your lab operations. Our solutions are meticulously designed to ensure full compliance with 21 CFR Part 11 regulations, offering robust electronic records protection and electronic signature capabilities.
Navigate the challenging landscape of FDA audits with the utmost confidence, knowing your data is precise, secure, and compliant.

FAQs
Lab Automation Solutions in the pharmaceutical industry play a critical role in enhancing efficiency, accuracy, and throughput by streamlining complex and repetitive lab processes. The lab solution-suite of Qualis LIMS, Logilab ELN, and Logilab SDMS helps automate high-volume tasks like sample preparation, compound screening, and data analysis.
Automation in the pharmaceutical industry enhances precision and reduces human error, contributing to more reliable outcomes and faster transition from research to clinical trials. In manufacturing, it guarantees consistent product quality and compliance with strict regulatory standards.
Logilab ELN is built for interoperability and can seamlessly integrate with existing SAP, ERP, Laboratory Information Management Systems (LIMS) etc, and other solutions commonly used in pharmaceutical labs using REST APIs. This integration capability facilitates efficient data exchange and process continuity, enhancing the lab's overall productivity and data coherence.
Qualis LIMS supports comprehensive quality control workflows essential for pharmaceutical manufacturing. Quality tests for huge batches of samples can be performed using configurable sample registration templates, sample life cycles can be tracked, non-conforming parameters can be flagged, and automated retests can be set up. These features help ensure that products consistently meet the required quality standards and regulations, critical for patient safety and product efficacy.
Absolutely. Logilab SDMS is designed to manage the large volumes of diverse data generated by high-throughput screening (HTS) technologies used in drug discovery. It can store, catalog, and make searchable vast datasets, enabling researchers to analyze results more efficiently and make informed decisions quickly. This capacity to handle big data is critical in identifying promising drug candidates early in the discovery process.
Qualis LIMS is specifically engineered to ease the burden of CFR Part 11 compliance in the pharmaceutical industry. It incorporates essential features like comprehensive audit trails, secure electronic signatures, and stringent user access controls.
These functionalities are critical for ensuring data integrity and security —- further, Qualis LIMS streamlines the creation of compliant reports, making it simpler for pharmaceutical companies to adhere to regulatory standards and reduce the risk of non-compliance issues.
Logilab ELN enhances collaboration in pharmaceutical labs by offering a digital platform for capturing and sharing research data, methods, and findings in real-time globally. Its secure access ensures data integrity and confidentiality, facilitating effective teamwork.
The system's ability to integrate with other laboratory software, like LIMS or SDMS, further enhances its collaborative capabilities. By streamlining workflow and data sharing, Logilab ELN not only improves teamwork but also accelerates the pace of research and development in the pharmaceutical sector.