Integrate seamlessly with over a thousand types of Analytical Instruments!
PC-Based
Capture and upload data from PC based instruments without restrictions on File Format or Size.
Standalone
Integrate with standalone instruments using RS232/TCPIP protocols based connectivity.
Clinical Diagnostic
Directly connect to Clinical diagnostic and analytical Instruments using Interfacer Middleware

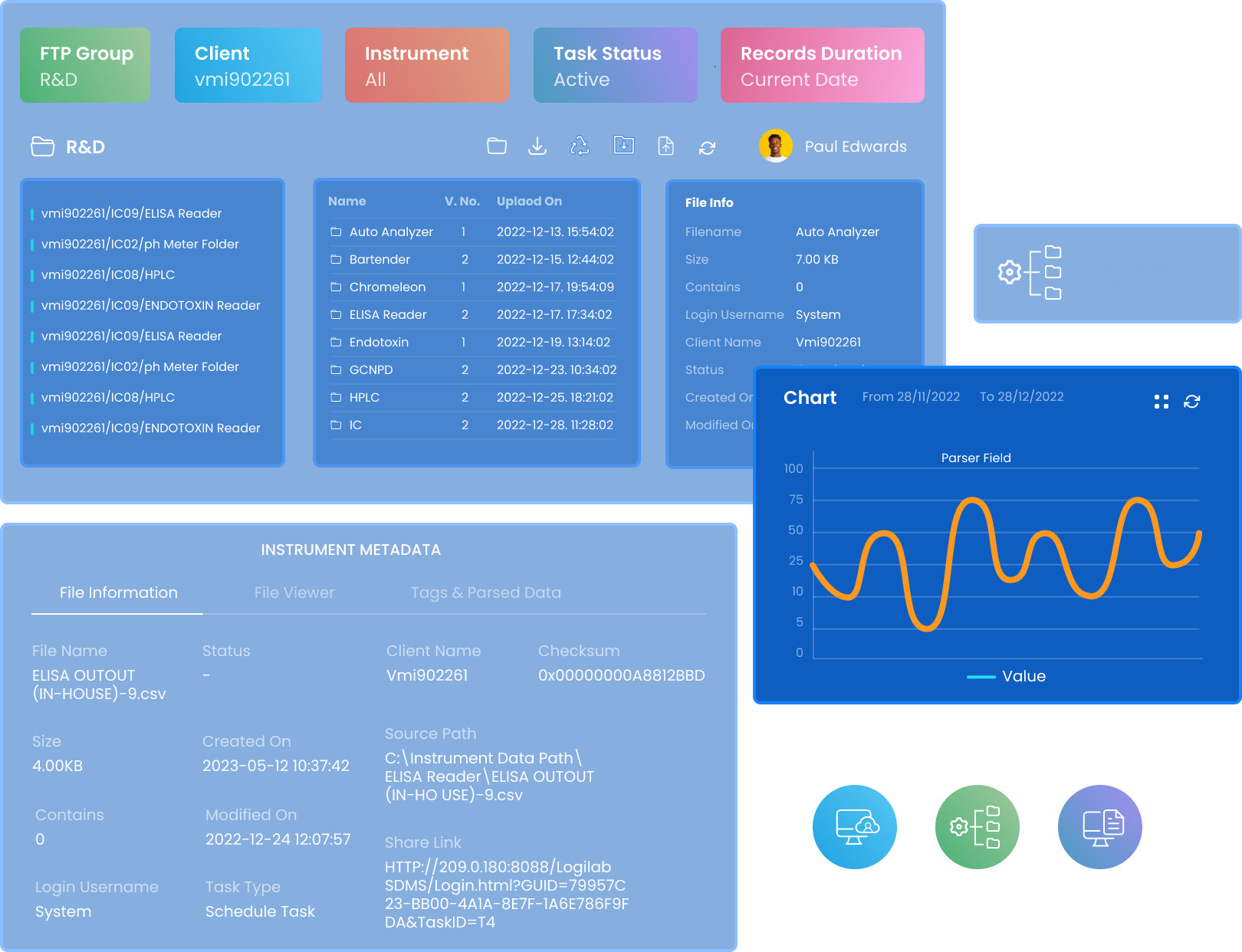
Designed for Enterprise Scale Scientific Data Management
Logilab SDMS, is designed to scale up and handle large volumes of data & has capabilities to capture data from thousands of instruments parallelly.
Instantaneous Data Capture
Automated data capture from diverse instruments in real-time, minimizing manual tasks and maximizing accuracy.
Cutting-edge Data Extraction
Our advanced parsing engine effortlessly extracts data of interest from Instrument Data Files, streamlining operations.
Highly Data Availability
Store your instrument's raw data and metadata in a secure FTP or Cloud server. Exchange data of interest with external systems such as LIMS,ELN, and more.
Make your data Findable, Available, Interoperable & Reusable
Discover the power of Logilab SDMS
Data Scheduler for Fully automated, Error-free data handling
Logilab SDMS features a powerful Data Scheduler which can perform Fully automated & error-free capture of Instrument data without human intervention.
Versatile Data Extraction
Capture data in real time or set up time-based scheduling in regular intervals.
Secure Data Storage
Store Instrument data and metadata in a secure FTP or Cloud server
Supervise Data flow
Monitor and track uploads and schedules in real-time.
See Full Instrument Compatibility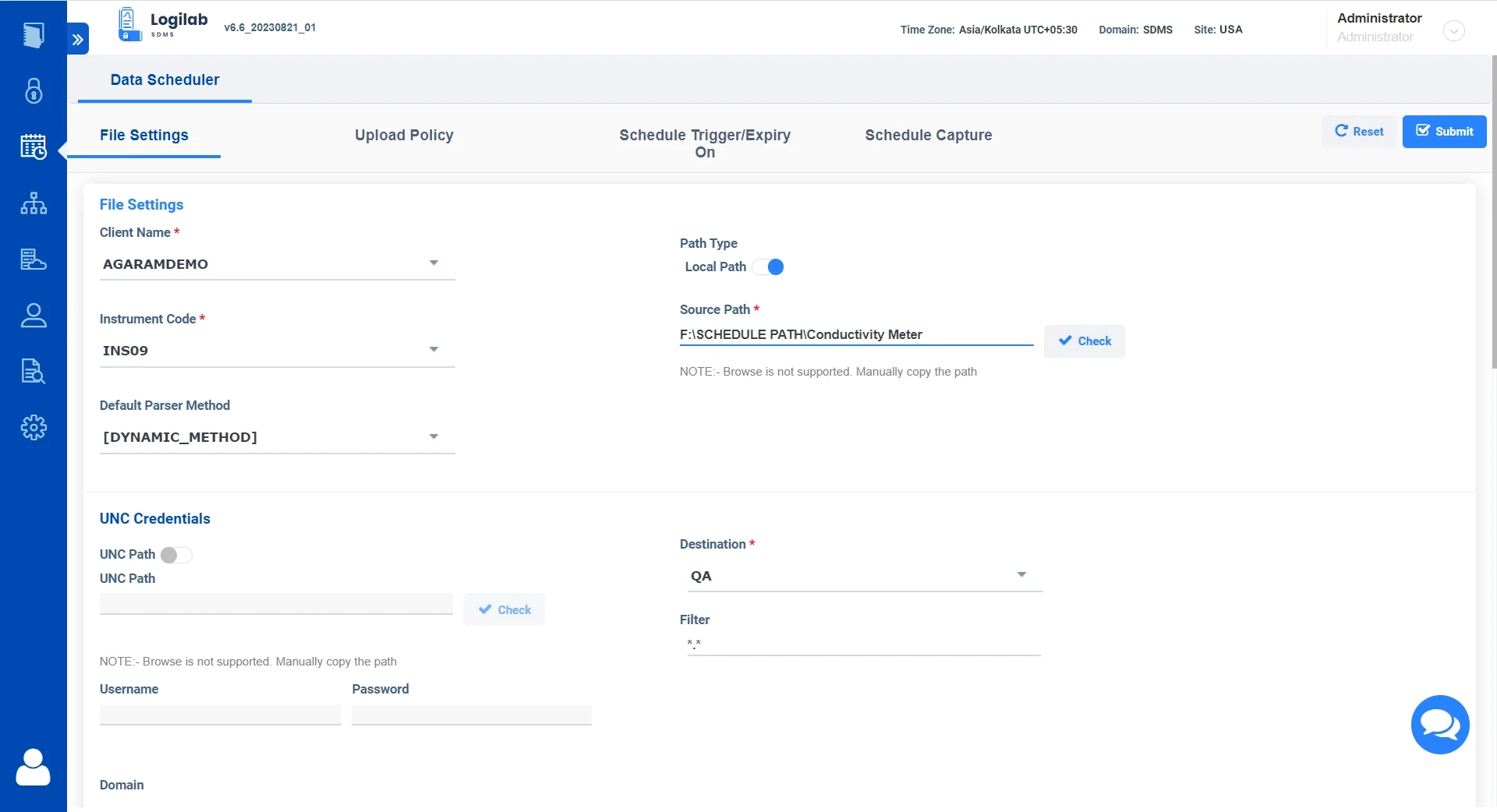
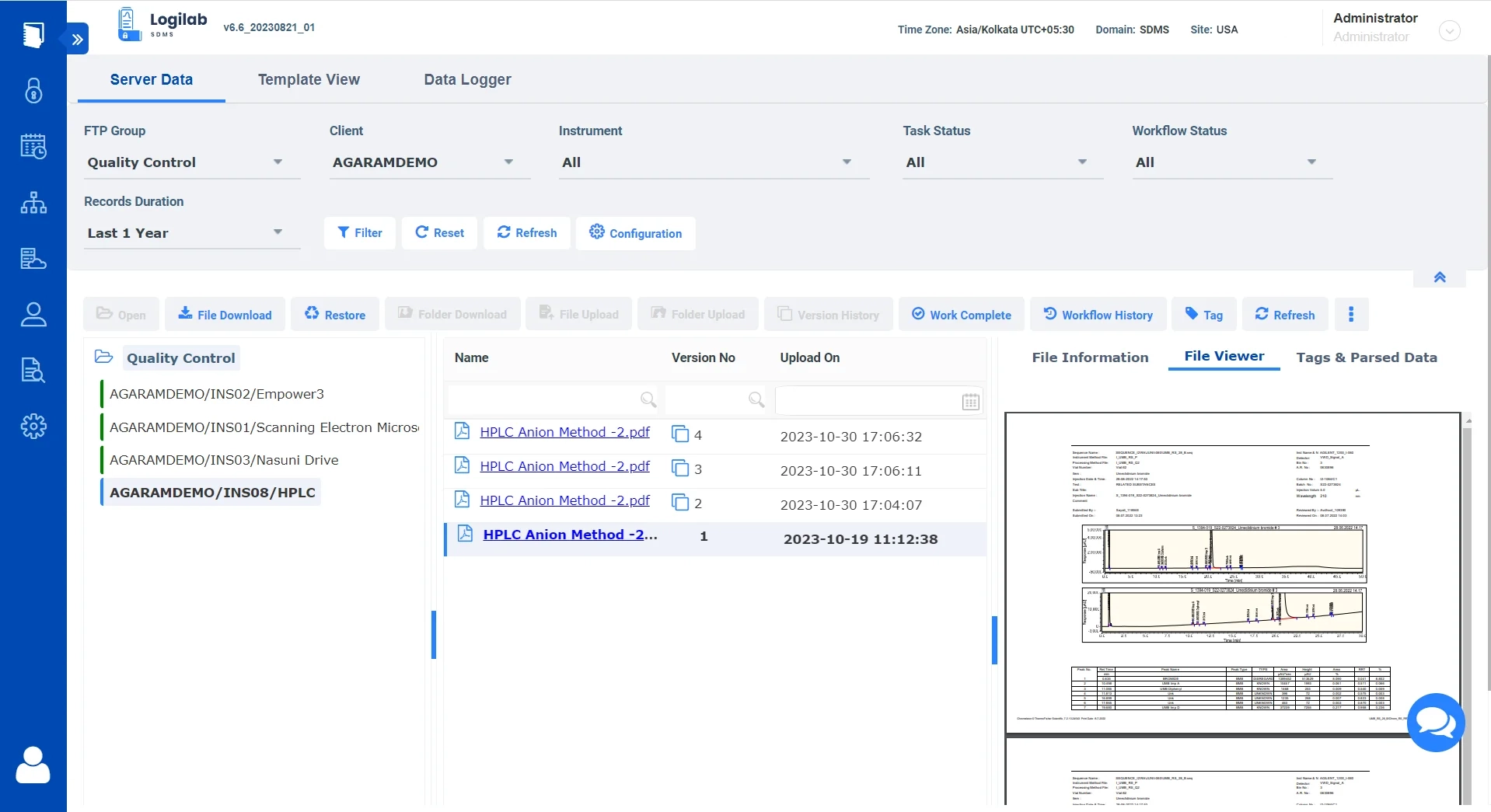
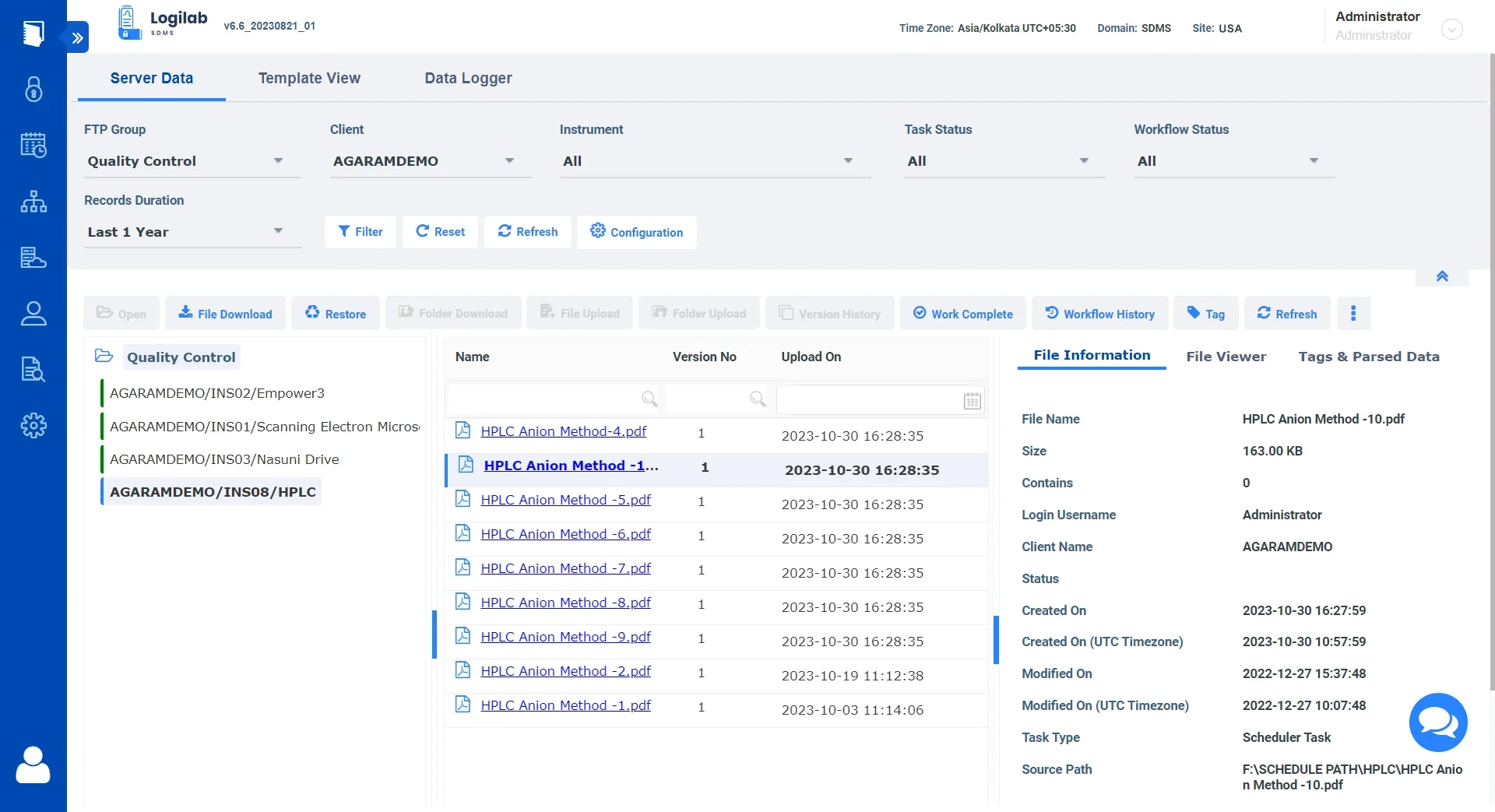
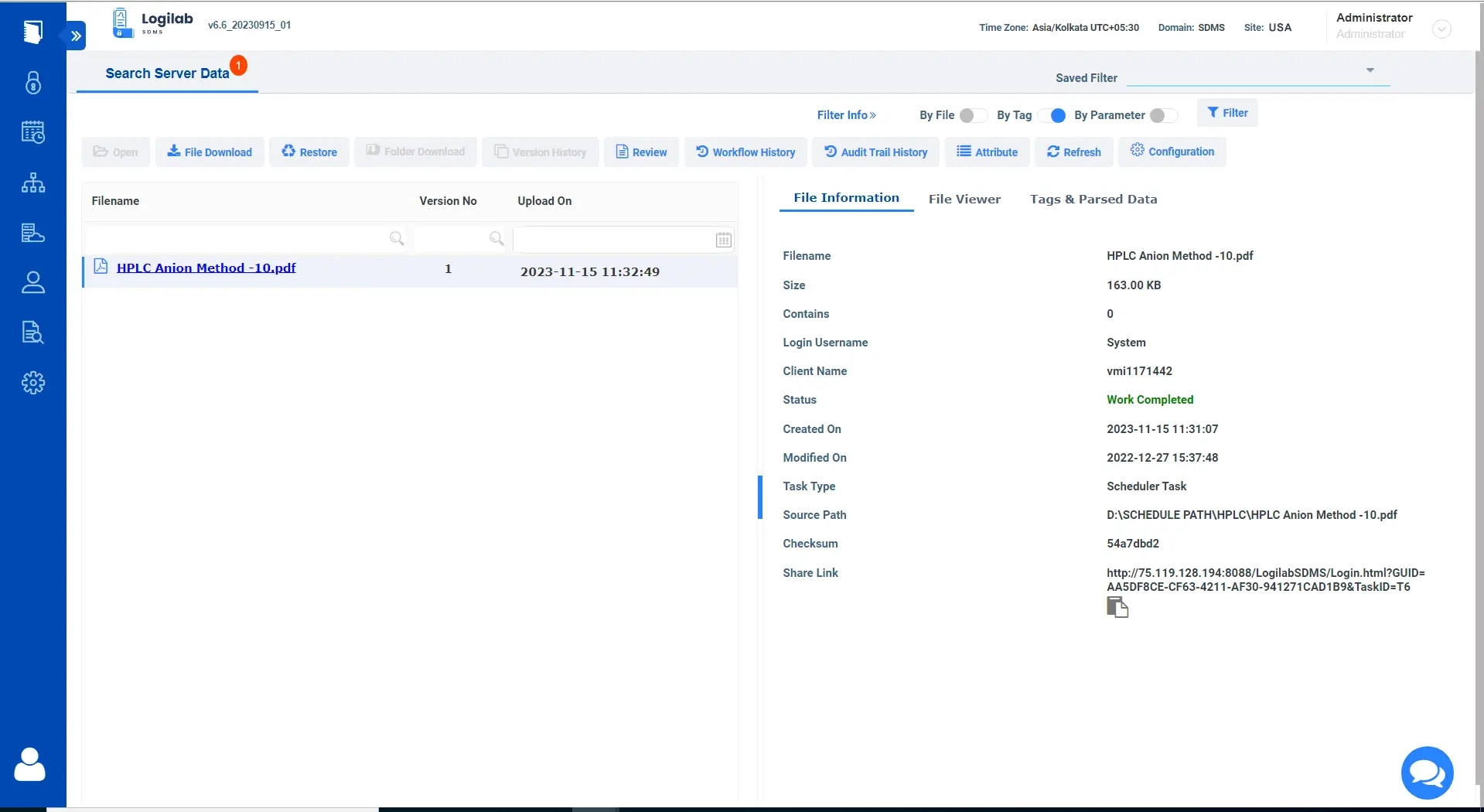
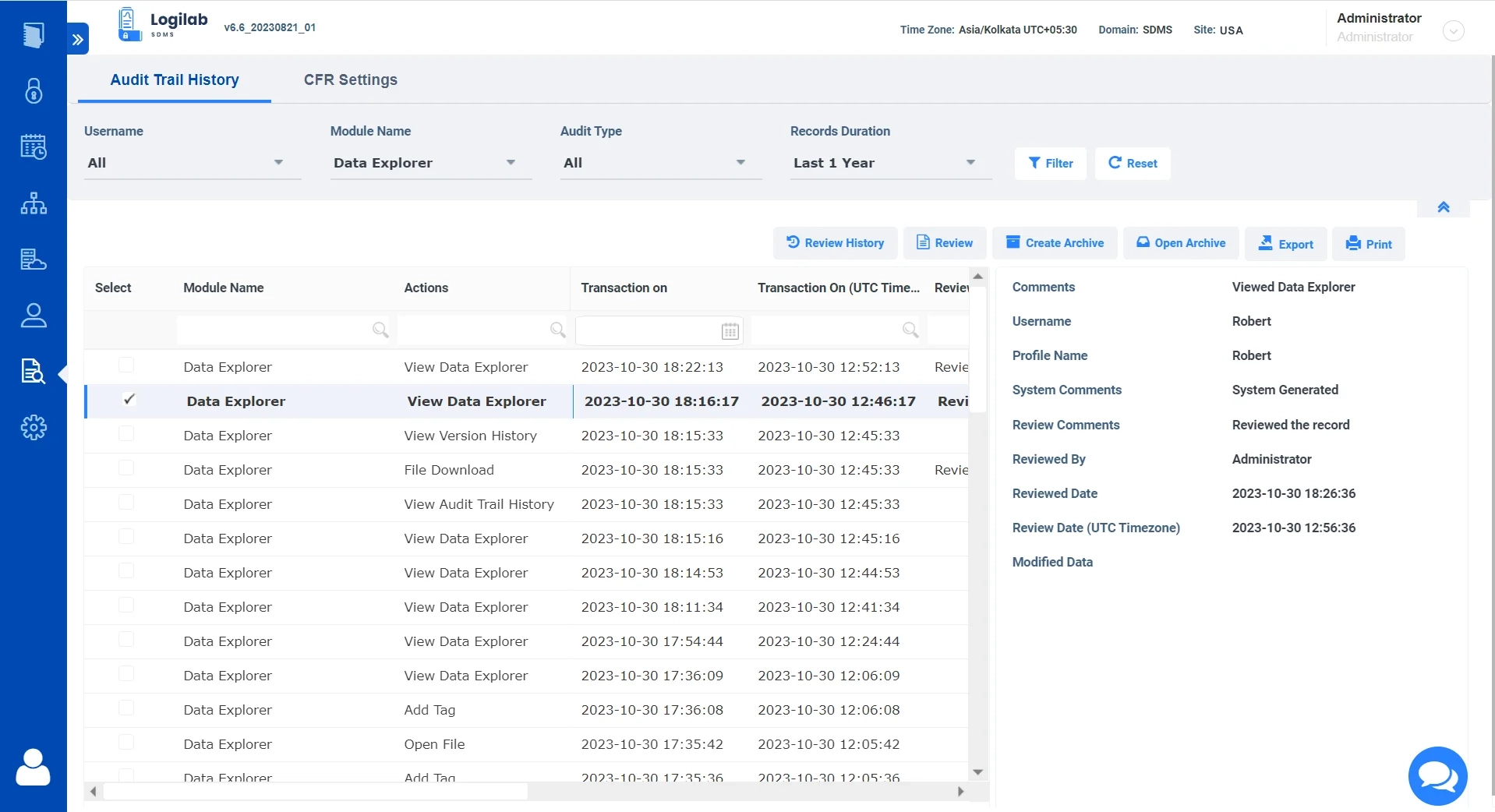
Data explorer for easy Navigation, Search & Management
Logilab SDMS features a full-fledged data explorer for organizing and viewing your scientific data
Built-in Data Explorer
Replicate the original folder structure of your local data within the file server for easy data organization.
Advanced Search
Comprehensive search and filters based on key parameters, tags and more.
Ensure Data Integrity
Complete Version control of all uploaded Files.
Simplified Data Format
View file details and content in a human-readable format
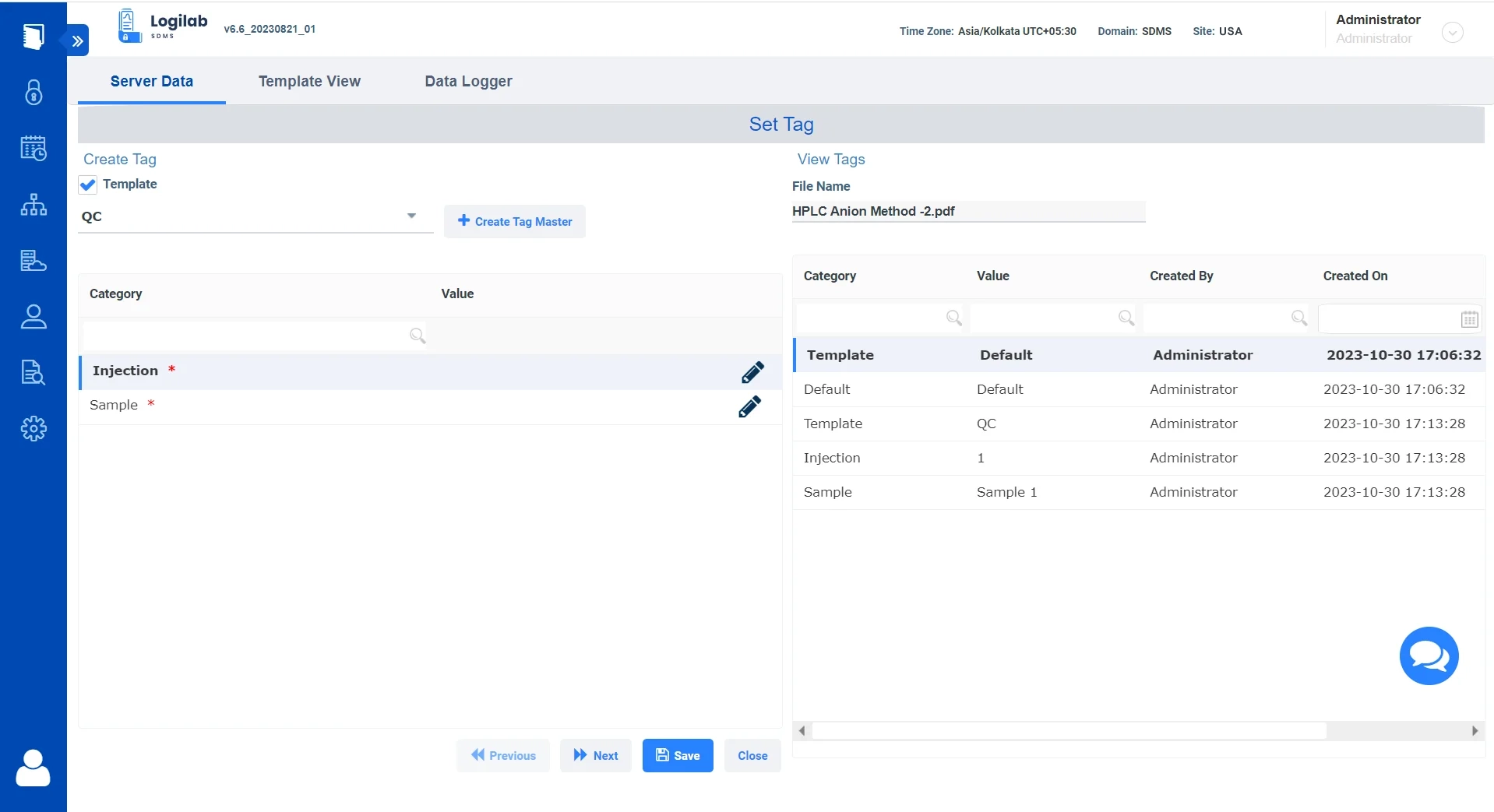
Download BrochureData Tagging for comprehensive organization & categorization
Auto- Index Data
Tag data to index and catalog files within the system.
Tag Metadata
Tag instrument data using metadata such as batch number, project, sample, and more for logical segregation, with pre- and post-tagging facilities that provide full flexibility.
Advanced Tag-Based Search
Quickly search through your instrument data based on tags
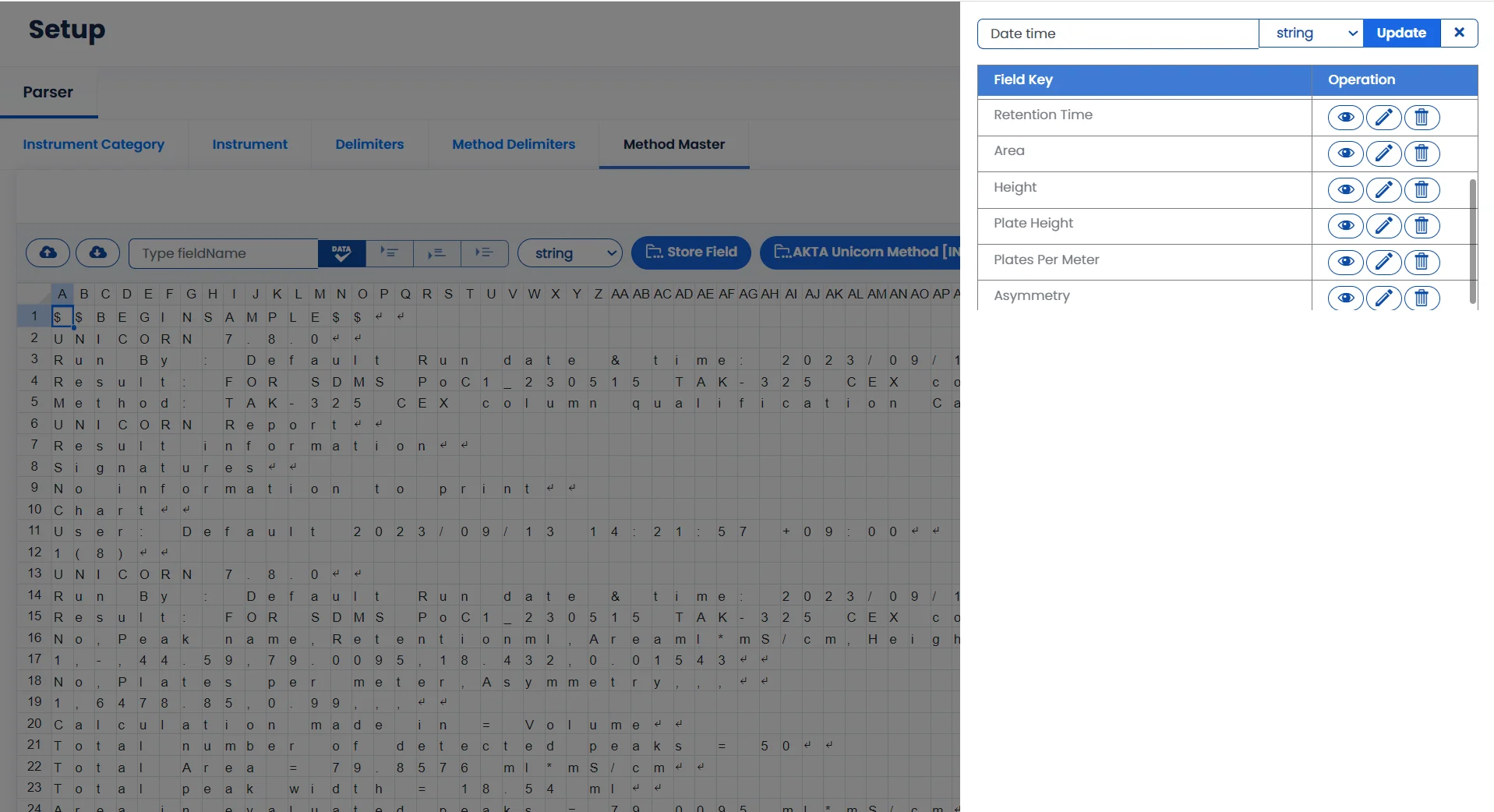
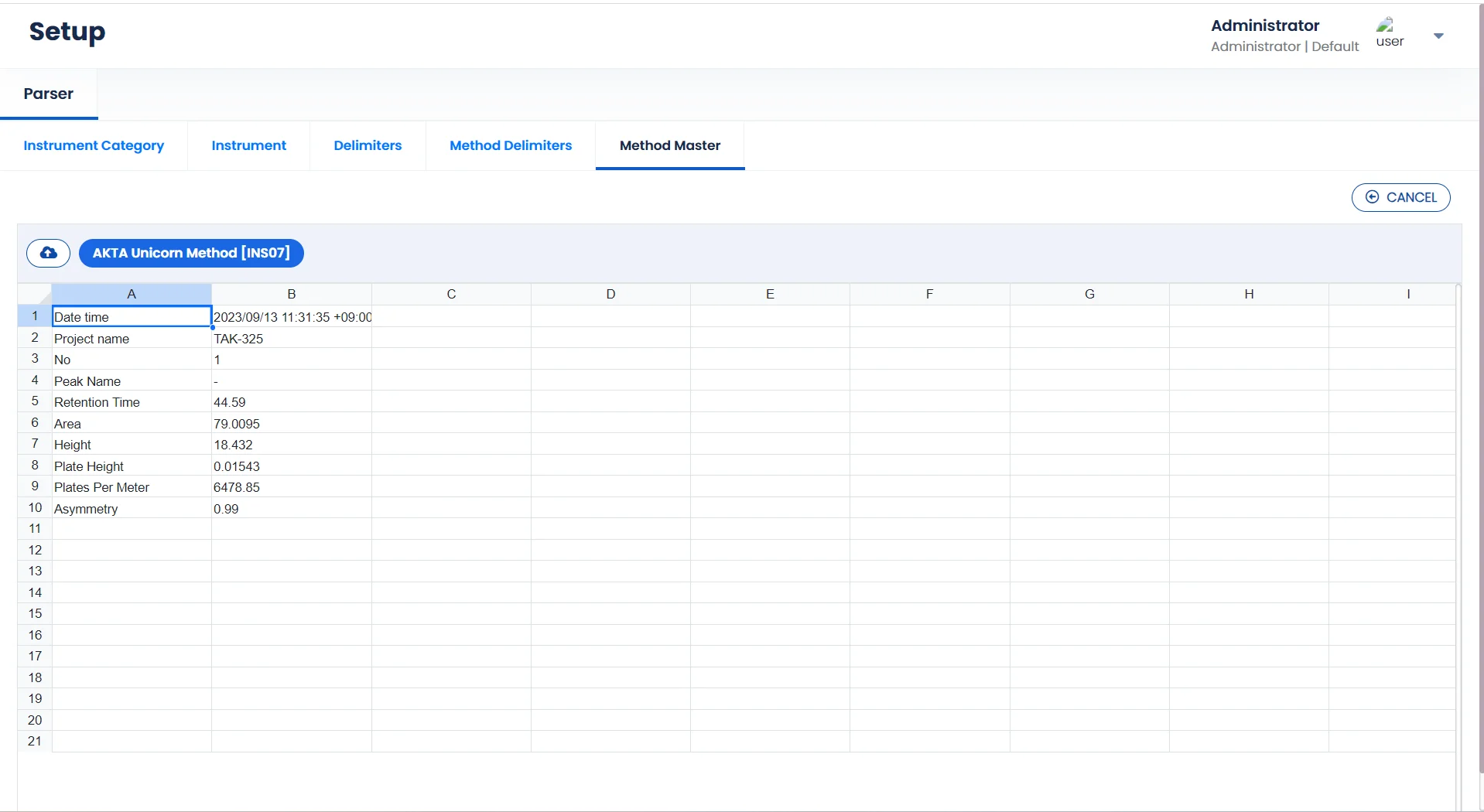
Contact SalesExtract key data points with our Advanced Parser
Logilab SDMS parsing engine automatically extracts data of interest from Instrument Data Files.
User-Friendly Interface
Easy-to-use interface for setting up data parsing logic
Gain Vital Insights
Extract key parameters from large volumes of instrument data
Intergate with External Systems
Send the extracted data to Qualis LIMS, Logilab ELN, or other external systems for data analytics.
Optimize Data Handling
Automate data processing and reduce human errors.
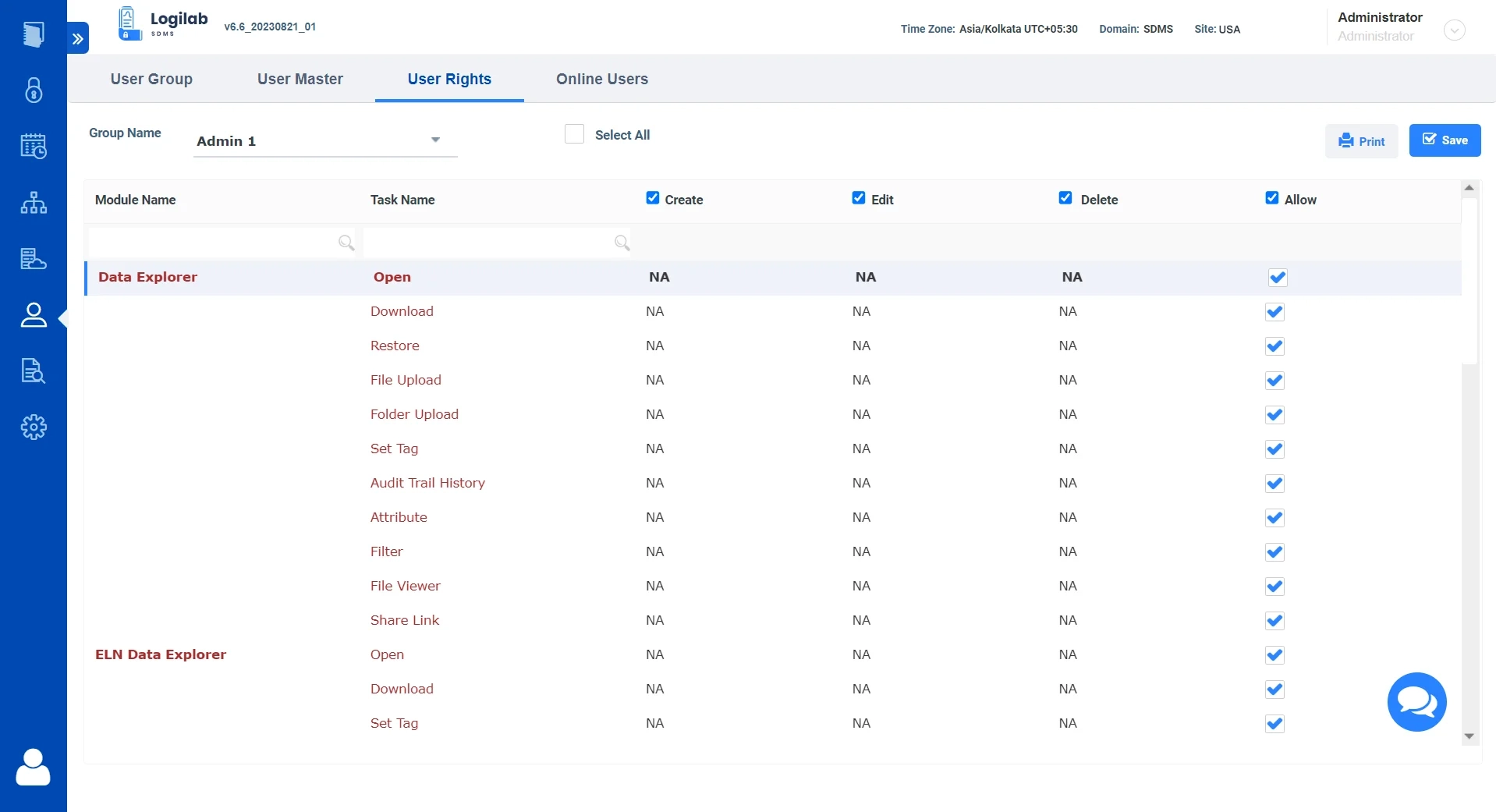
Read Case StudyAccess Control & Traceability of Data for Regulatory Compliance
Enable Controlled Data Access
Role and rights-based access to data within the syste
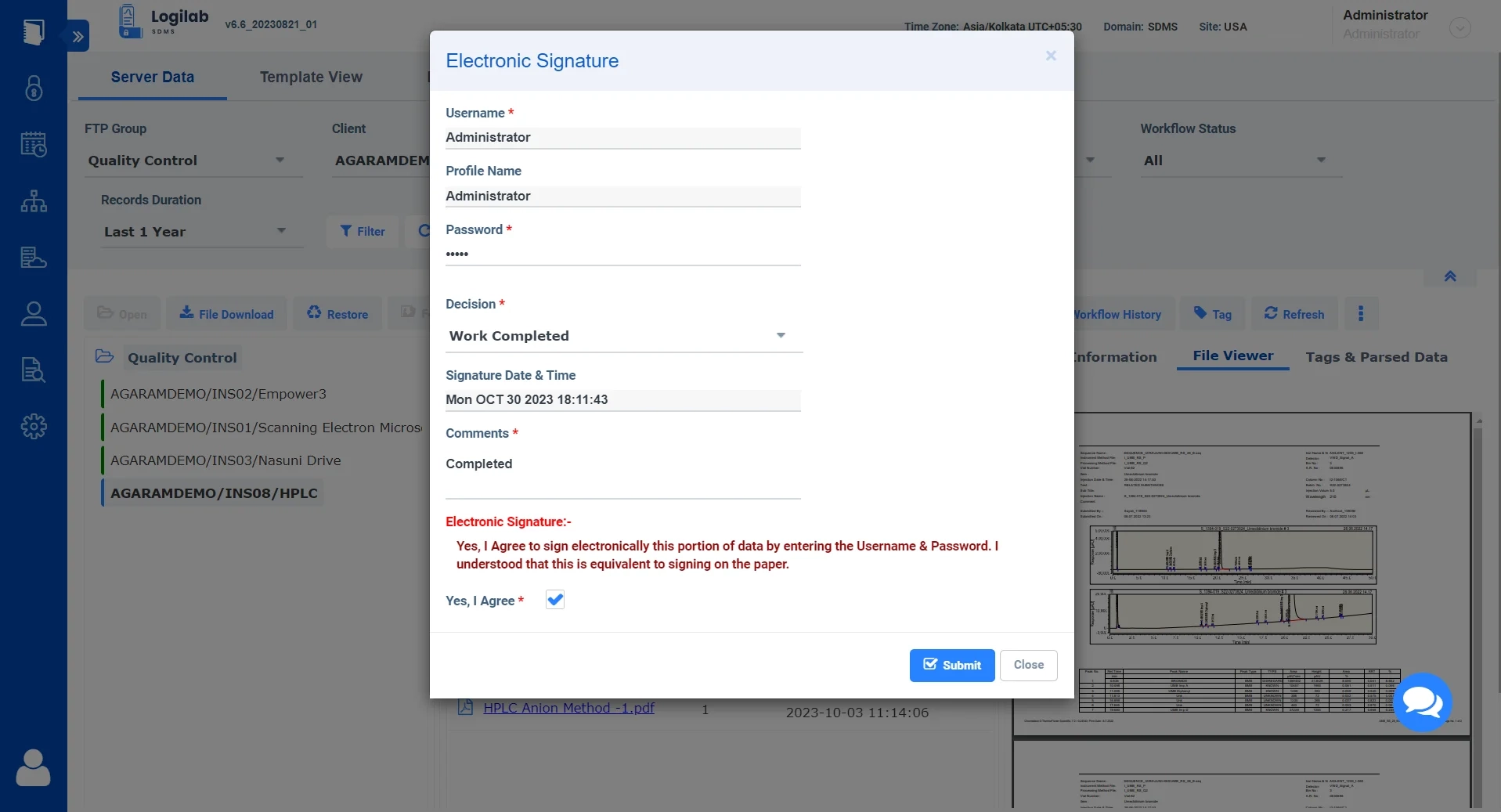
Review Workflow with E-Signs
Ability to use workflow to review and approve with electronic signature for better compliance
Adhere to Regulatory Compliance
Audit trail and logging systems for 21 CFR Part 11 compliance.
Make Non-Compliant Instruments Compliant with CFR Gateway
CFR Gateway has been designed to provide 100% data integrity and compliance for instrument software applications and standard desktop applications such as Microsoft Office.
Upon Operating System boot,the CFR gateway acts as a secure portal through which controlled access to system functions and applications can be enabled for authorized users.
Any data created or modified by the user is fully tracked and sent to a central repository automatically.
This enables instruments that do not have compliance-related features, comply with CFR part 11 and Data integrity guidelines.

Logilab SDMS is the #1 Choice of Industry Leaders
Championed by top-tier Pharma and Life Sciences entities, Logilab SDMS isn't just a tool—it's a transformative experience in data management. From capture to analytics, we have redefined scientific data management for customers across the globe





Customer Success Stories
Learn how Logilab SDMS helped Moderna Therapeutics achieve Data Integrity, Compliance and automation across their Manufacturing plant QC and research Labs.

How Logilab SDMS helped a COVID vaccine manufacturer achieve Data Integrity, Compliance & Automation
A leading Biopharmaceuticals organization that is an early innovator of the COVID-19 vaccine wanted to achieve an automated process for achieving data integrity and compliance...

Dive Deeper into Logilab SDMS
Ready to redefine your data management paradigm? Explore our Brochure or Request a Demo to witness the Logilab SDMS magic first-hand.

Would you like to learn more about Logilab SDMS?
Email Us: [email protected]