Blogs
Stories on culture, tech, teams, and tips.
Featured Article of the Month
 General
General SDMS vs. File Storage: Why Labs Need Intelligent Data Management
In the era of big data and digital transformation, laboratories are experiencing an unprecedented surge in the volume, complexity, and...
by adminMay 6, 2025
Most Popular Articles
Recent Posts

How Qualis LIMS Helps Laboratories Comply with 21..
by adminSeptember 27, 2024
Transforming Data Management in Labs: An Introduction to..
by adminSeptember 18, 2024
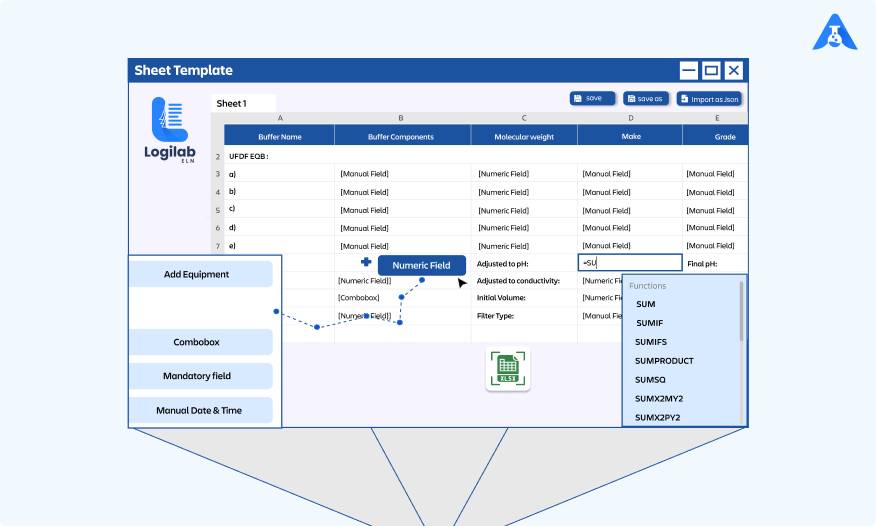
Streamlining Scientific Excellence: Unveiling the Power of Logilab..
by adminSeptember 3, 2024

Most Popular Articles
Get Started With our Products,
ask for a demo
Seeing is believing, reach out us for a presentation & demo





