In the dynamic and intricate world of life science and biotechnology laboratories, adopting advanced systems is pivotal in addressing the unique challenges these fields face. From managing vast and complex datasets to ensuring stringent regulatory compliance, and from enhancing accuracy and reliability in experiments to facilitating seamless collaboration across global teams, digital solutions are transforming the landscape of scientific research. They are instrumental in driving efficiency, fostering innovation, and maintaining a competitive edge in the fast-paced and ever-evolving realms of life sciences and biotechnology.

Comprehensive Laboratory Management
Sample Tracking and Management
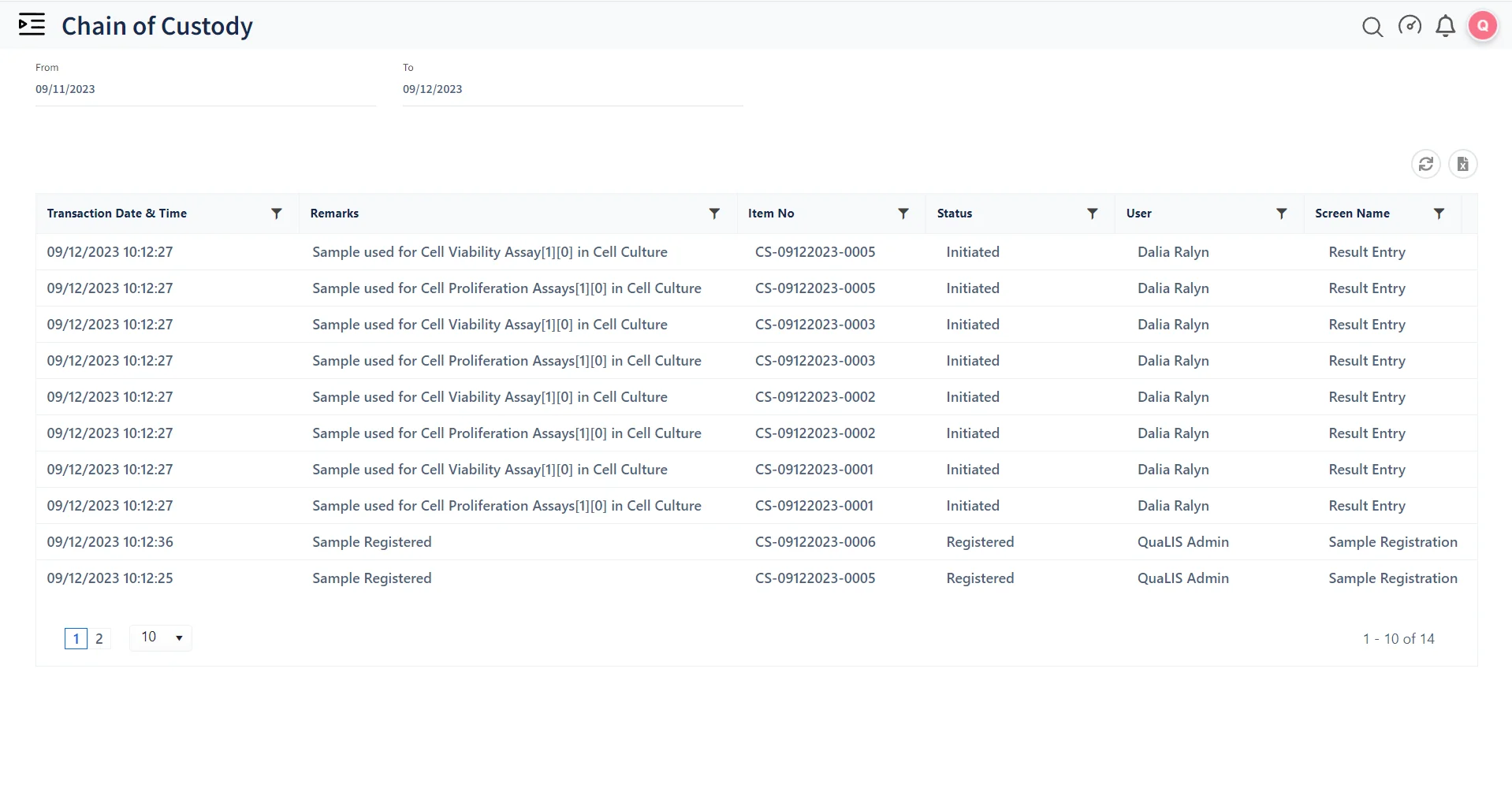
Manage managing large volumes of samples in life sciences. Tracks each sample's lifecycle, from collection to testing and storage, ensuring accurate and efficient handling.
Quality Control and Compliance
Qualis LIMS (Laboratory Information Management System) helps laboratories adhere to industry standards and regulatory requirements. Ensures that all processes are documented and auditable, which is crucial for compliance with regulations like FDA (Food and Drug Administration) (Food and Drug Administration), GLP (Good Laboratory Practices), GMP (Good Manufacturing Practices), and ISO.
Enhancing Life Sciences with Digital Efficiency and Compliance
Digital Record Keeping
Labsheets of Logilab ELN (Electronic Lab Notebook) replace traditional paper notebooks, allowing scientists to record experiments digitally, leading to better organization, searchability, & data sharing across teams.
Compliance with regulatory standards like 21 CFR Part 11
Audit trails, version control, and electronic signatures, ensures compliance and makes your data ready for publication, patent applications & regulatory submissions.
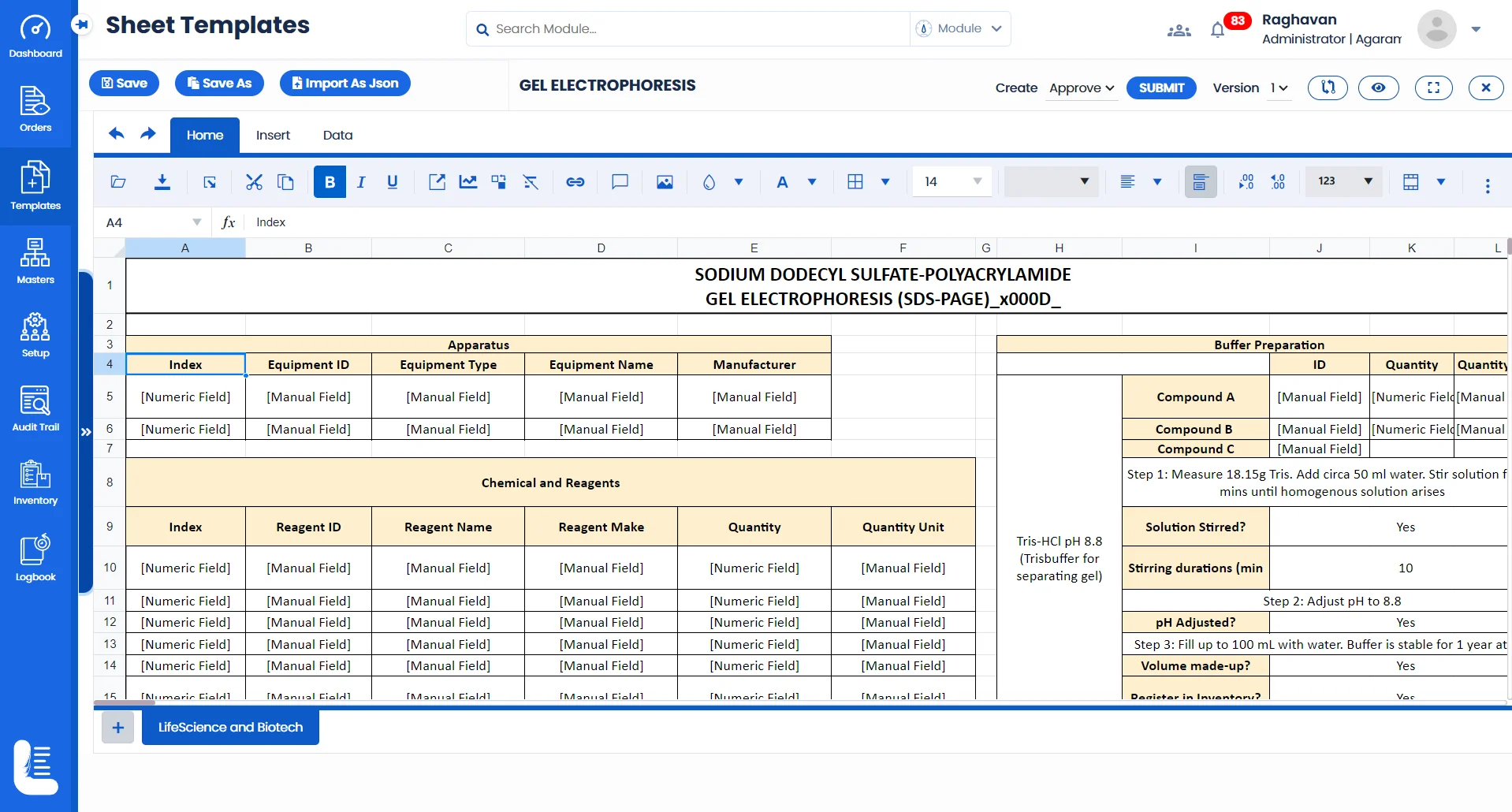
Streamlined Protocol and Task Management
Protocol-based templates and task management ensures that all steps of an experiment, from hypothesis to conclusion, are meticulously recorded and executed, which is essential for reproducibility and validation of scientific research.
Optimizing Data Capture, Integration, and Instrument Flexibility
Real-time Instrument Data Capture and Management
Capture data in real-time from critical experiments, such as those involving live cell cultures, genetic sequencing etc to be accurately captured and logged with accuracy.
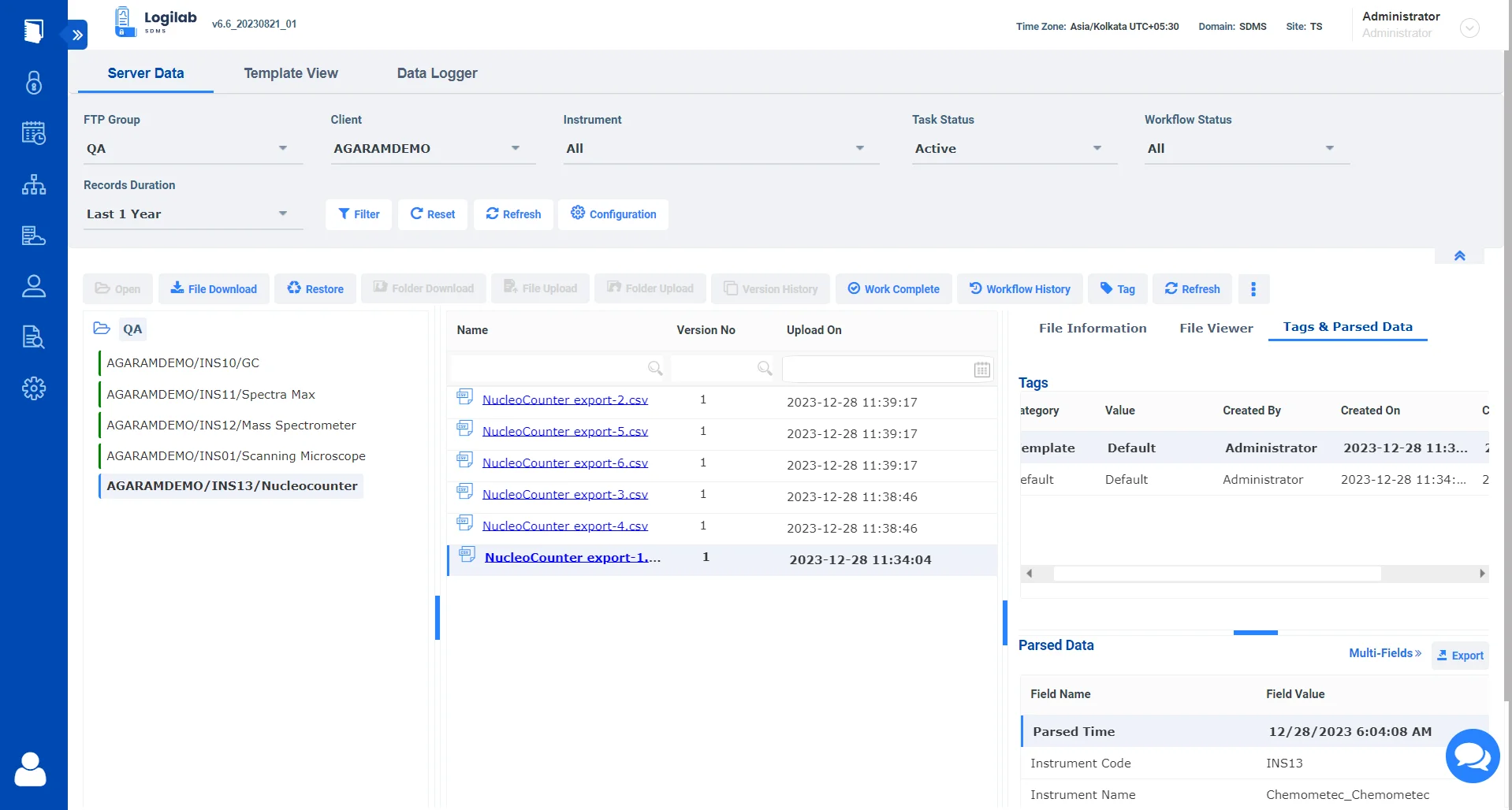
Unified Data Hub with Advanced Data Extraction
Consolidate and manage data from diverse sources, including chromatography, mass spectrometry, and molecular biology instruments. In fields where data volume and complexity are significant, advanced data extraction capabilities are useful for parsing complex datasets.
Scalability and Versatility for Diverse Instrument Integration
Integrating with a wide range of instruments, including specialized and high-throughput devices, ensures that labs can adapt to evolving research needs without being constrained by data management systems.
FAQs
In the Life Sciences & Biotech Industry, digital labs such as Qualis LIMS are generally more cost-effective in the long run. They simplify sample management, boost the consistency of experiments, and minimize expensive compliance mistakes.
Qualis LIMS enhances resource use, reduces the need for physical storage for paper-based record keeping, and can greatly accelerate research and development processes.
Lab Automation Software in life sciences and biotech labs includes tools like Qualis LIMS and Logilab SDMS. These systems automate routine lab tasks, such as sample tracking, instrument integration , data capture, and analysis.
This automation reduces human errors, increases efficiency, and frees scientists to concentrate on more complex analytical work. These benefits contribute to enhanced productivity and accuracy in laboratory settings.
In life sciences research, data integrity is paramount. Qualis LIMS helps ensure data integrity by providing a structured framework for data capture, storage, and retrieval. It records a detailed audit trail for the lifecycle of each sample and associated data, thus ensuring that all processes can be tracked. Features like electronic signatures, role-based access controls, and automated data backup further strengthen data integrity and support compliance with regulatory standards such as FDA 21 CFR Part 11.
Yes, Logilab SDMS is designed to handle complex and diverse data types generated by a variety of laboratory instruments. It can capture, catalog & archive instrument data from different sources, enabling better data analysis and visualization.
By interfacing with high-throughput instruments and analytics platforms, Logilab SDMS can manage raw data, metadata, and results, facilitating advanced data mining and interpretation, which are crucial in life sciences research for meaningful insights.
Qualis LIMS and Logilab SDMS enhance collaboration by enabling centralized data storage and secure sharing of information across different departments and globally distributed sites. They support remote access, allowing researchers to access data and contribute to projects from any location.
These systems also enable role-based access control and real-time data sharing, which are essential for collaborative biotech projects that may involve multiple stakeholders, including external partners and contract research organizations.