Public Health laboratories normally implement a LIMS as the first solution as it involves quick release of patient sample results. These laboratories are focused on high throughput results but at the same time need to be careful about the quality of the results published.
Qualis Laboratory Information Management System helps Hospitals, Clinical Labs, and referral diagnostic laboratories in achieving operational excellence.

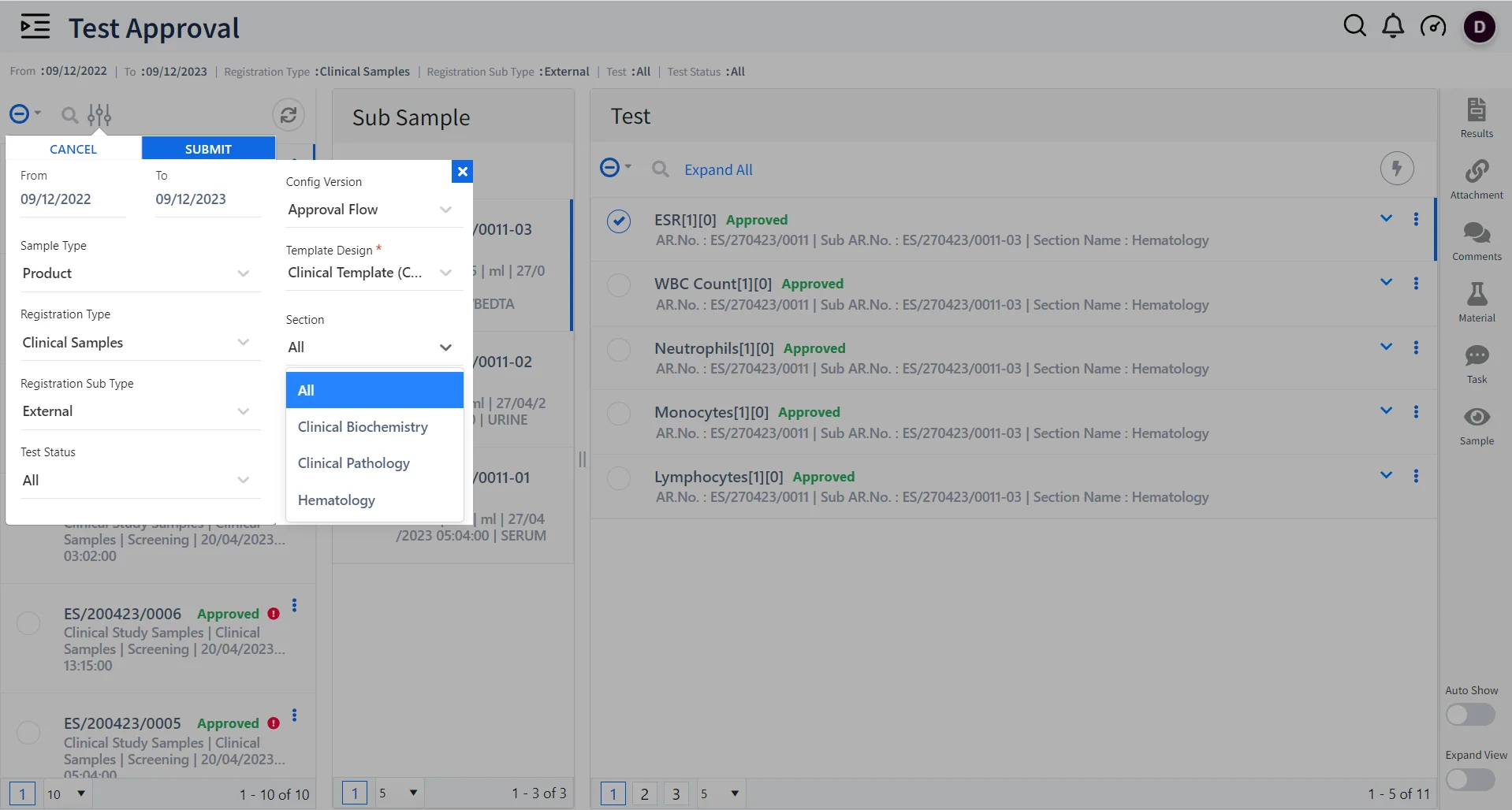
Dynamic Sample and Test Management using Qualis LIMS
Multi-section testing
Qualis LIMS acts as a single secure system to execute tests for samples across multiple sections such as hematology, biochemistry, immunology, histopathology, microbiology, etc.
Sample data collection and reconciliation
Collect and receive sample data from globally distributed labs and collection centers into one central database for testing.
Enhanced Patient Data Management
Conduct normal & batch tests on large volumes of patient samples and obtain accurate results; Automate routine tests through Dynamic registration templates based on patient type and sample type.
Generate Test Reports
Generate large volumes of patient test reports based on various test parameters ensuring timely delivery.
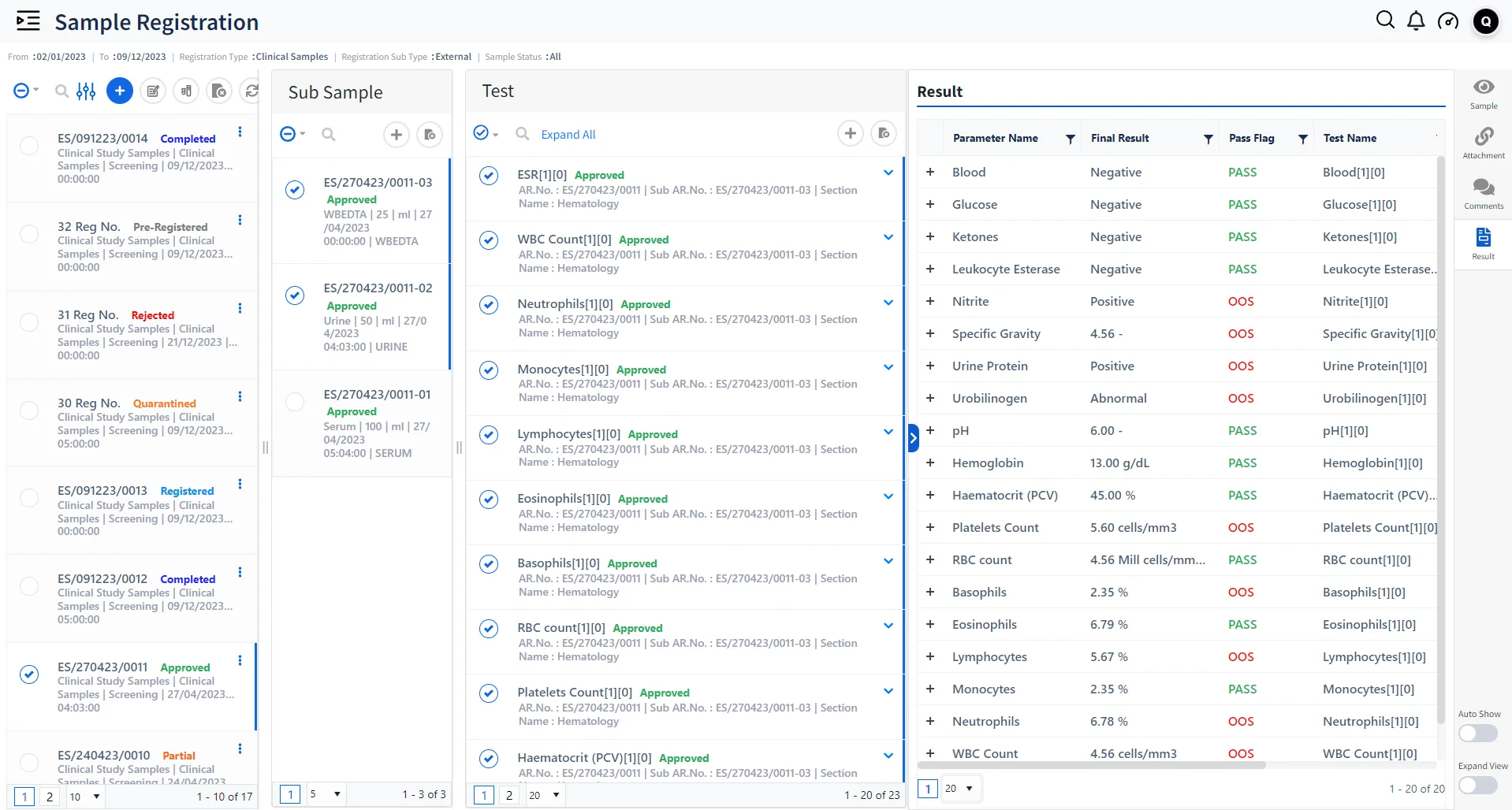
Enhancing Healthcare Efficiency and Compliance:
Streamlined Data Management and Collaboration
A centralized platform for managing all laboratory data, including patient records, test results, and research notes. Can integrate with other hospital and laboratory systems (like LIMS and HIS (Hospital Information System)) ensuring seamless data flow and improving overall efficiency in patient care and research.
Enhanced Compliance and Audit Trails
Maintain compliance with standards like HIPAA, CLIA (Clinical Laboratory Improvement Amendments), and FDA (Food and Drug Administration) (Food and Drug Administration) regulations. Provides robust audit trail capabilities, documenting all changes, access, and data handling in a secure and traceable manner. Ensures data integrity and reliability, which is vital for patient safety and quality control in healthcare laboratories.

Data Capture from Clinical Diagnostic Instruments
Interfacer Middleware is a configurable middleware solution that automates capturing data capture by extracting and parsing data from Clinical Diagnostic Instruments ensuring that healthcare professionals have immediate access to critical data for analysis and decision-making.
FAQs
Qualis LIMS is crucial for Healthcare & Diagnostics labs, streamlining patient sample management from collection, testing to disposal. It provides a configurable template based approach for testing patient samples based on the sample type and disease being tested. The system ensures detailed traceability for each sample, vital for patient safety and diagnostic accuracy. Its traceability features uphold accountability and enable transparent, accurate reporting, aligning with healthcare regulations and ensuring effective patient care.
Interfacer Middleware acts as a bridge between clinical IVD instruments and scientific data management systems like Logilab SDMS, enabling seamless communication and data transfer. It has open communication protocols and an advanced parsing engine to interpret and standardize data from various diagnostic instruments, automating the data capture process, reducing manual entry errors, and ensuring timely and accurate test results are available for labs.
Interfacer Middleware streamlines diagnostic testing workflows by automating the transfer of data between clinical laboratory instruments and LIMS or other external systems. It reduces the time spent on manual data entry, minimizes the risk of transcription errors, and allows for quicker processing of diagnostic tests, thereby improving the overall turnaround time for delivering patient results.