Contract research laboratories face numerous challenges, given the complex nature of their work, which includes efficient management of diverse samples to maintaining stringent data integrity and compliance with ever-evolving industry regulations. Labs also grapple with the need for to maximize efficiency to ensure timely project delivery.
The complexity further extends to streamlining collaboration among geographically dispersed teams, as well as proper project management for quick and easy execution of tests and assays for clients. To address these challenges, Agaram’s lab informatics solutions are designed to seamlessly integrate into existing CRO workflows, enhancing overall operational efficiency and reliability.
Mastering Sample and Data Management
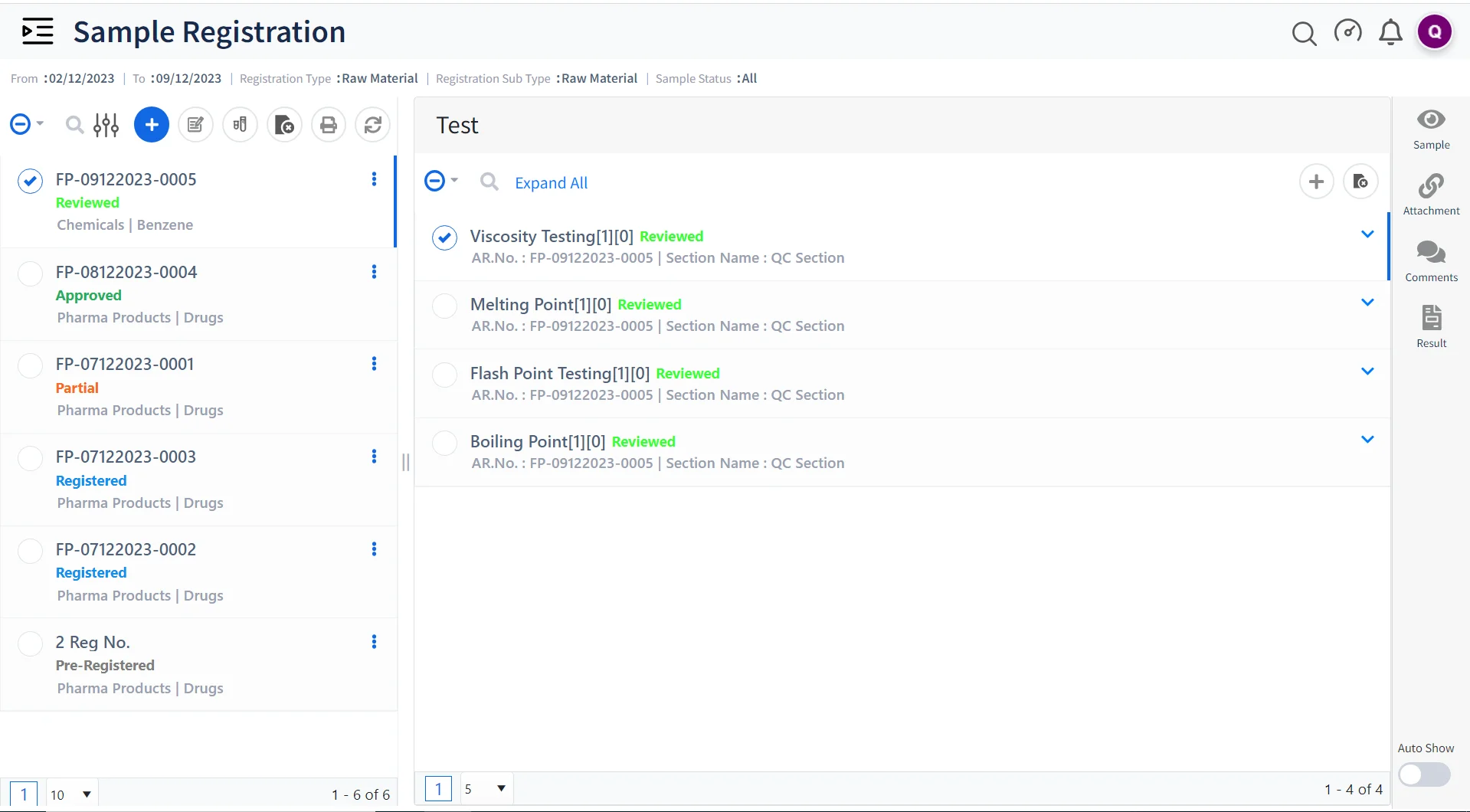
Sample Management and Tracking
Qualis LIMS streamlines the management of samples, their storage, and the associated data, ensuring that contract research labs can easily track and trace samples, raw materials, etc throughout the lifecycle of the project.
Elevate Quality Control Standards
Maintain strict quality control by setting up protocols and generating comprehensive reports, ensuring that the results meet quality standards and can be easily shared with clients and regulatory authorities.
Create Dynamic Reports
Qualis LIMS provides a built-in powerful reporting tool for labs to design their custom reports based on project clients or sample.
Seamless Collaborative Research
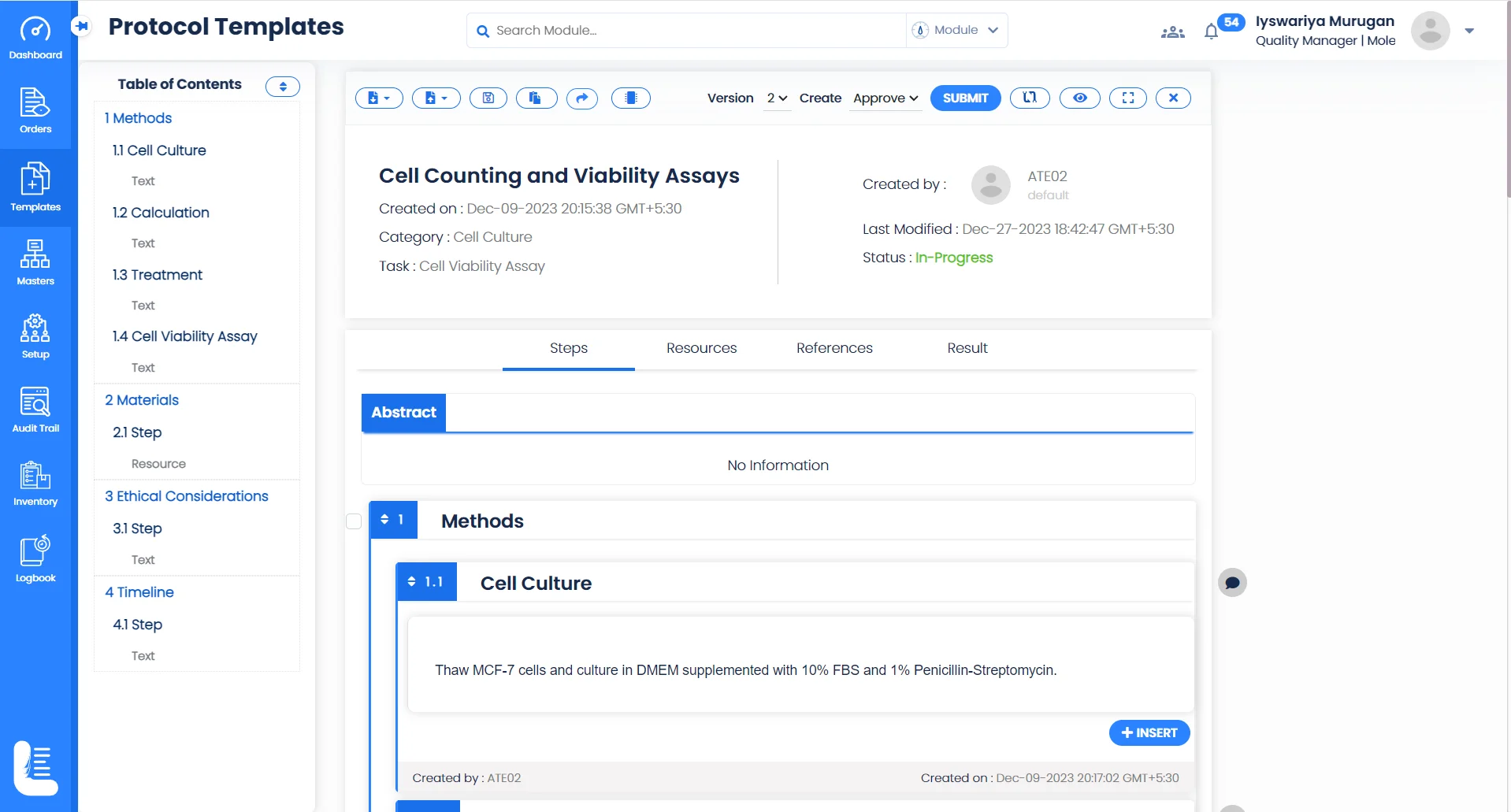
Electronic Experiment Records
Create and store experimental and research data electronically, making it easy to record observations, methods, and results, reducing the risk of data loss and enabling easy retrieval for analysis or audit purposes.
Collaborative Research
Logilab ELN provides an advanced folder-based interface for creating and managing projects and tests, ensuring that everyone involved in a project can access and contribute to experimental data, enhancing transparency and efficiency.
Workflow Automation
Create customizable workflows to create, manage, and monitor processes, such as sample analysis, in a highly efficient and standardized manner, reducing errors and speeding up project timelines.
Efficient Document Management
Streamlined Research Document Management
Qualis DMS simplifies the management of documents and data generated during research projects, including regulatory documents and reports making it easier for contract research labs to organize, secure, and retrieve essential documents.
Document Version Control
Automated version control provides easy access to the latest approved documents, SOPs, Safety Protocols and more.
Document Retrieval and Accessibility
Ensure quick and secure retrieval of critical documents through a user-friendly interface.
Streamline your labs project management today
By leveraging Qualis LIMS, Logilab ELN, and Qualis DMS, contract research labs can enhance efficiency, maintain data quality, ensure compliance, and streamline their research processes, ultimately delivering higher-quality results to their clients.

FAQs
Adopting Logilab ELN is cost-effective for small CRO labs. It offers a flexible, scalable solution for managing diverse project data and documentation. By digitizing lab notebooks, it cuts costs on physical storage and manual records. Improved data organization and retrieval streamline workflows, saving time and resources. This efficiency and enhanced client service make it a smart investment for budget-conscious labs. Logilab ELN also ensures high-quality, traceable data, meeting client and regulatory standards - vital for sustaining business and reputation in the competitive CRO industry.
Qualis LIMS provides Contract Research Organizations with an efficient sample management system that tracks samples from receipt through disposal, ensuring complete traceability. It automates sample registration, labeling, storage, and retrieval processes, which helps CROs manage high volumes of samples for multiple clients. This traceability is essential for CROs to maintain accountability and provide transparent reporting to their clients.
Qualis LIMS also enables CROs to generate certificates of analysis based on client data with ease. The system's integrated reporting tool allows for the generation of customized reports tailored to specific client requirements as well.
Logilab ELN is designed to facilitate collaborative research efforts among teams distributed across different locations. It allows real-time data sharing and project updates, which is crucial for CROs working with various clients and partners. The ELN supports secure access controls and audit trails, ensuring that intellectual property is protected while enabling seamless collaboration.
Answer: Yes, Qualis DMS is capable of managing the wide array of data types generated during preclinical and clinical trials. It offers a centralized system for storing and organizing documents, protocols, results, and reports. This system supports version control and searchability, which are vital for managing the extensive documentation involved in trials and ensuring quick retrieval during audits or reviews.
Qualis LIMS is built with data integrity as a core principle. It features electronic signatures, audit trails, secure access controls, and automated data backup to ensure traceability and meet regulatory compliance standards.
Qualis DMS is designed to be agnostic to the type of document that is being uploaded or managed. It supports various file formats, version control, and secure access controls to manage and distribute different types of documents efficiently. You can easily search, archive, and retrieve documents throughout the entire research lifecycle.
Yes, Qualis LIMS offers configurable workflows for document review and approval, streamlining the process and ensuring adherence to internal protocols and client requirements. This saves time and reduces errors associated with manual document routing.