In the era of big data and digital transformation, laboratories are experiencing an unprecedented surge in the volume, complexity, and significance of scientific data. Whether in pharmaceutical, chemical, clinical, environmental, or research domains, modern labs are no longer just centers of experimentation—they have evolved into data factories.
But with more data comes greater responsibility: How do you store it, retrieve it, trace it, protect it, and ensure its accessible, authentic, reliable, and audit-ready? Traditionally, many labs have relied on basic file storage solutions—SharePoint drives, Dropbox, Google Drive, or local servers. While familiar, these systems are often stretched far beyond their limits in scientific settings.
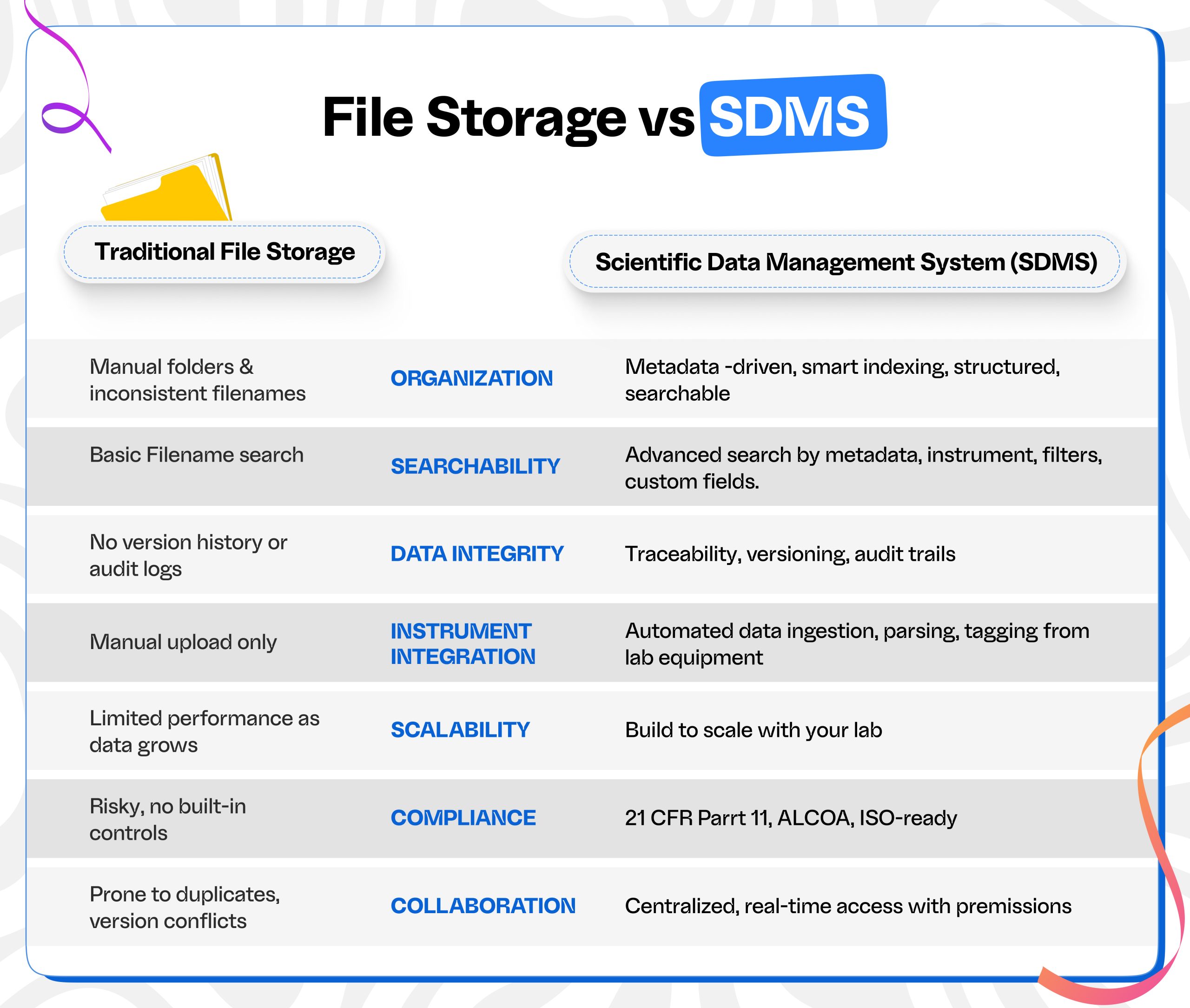
This is where Scientific Data Management Systems (SDMS) enter the picture. Unlike conventional file storage tools, an SDMS is purpose-built to handle the unique needs of labs: managing structured and unstructured data, linking instrument raw data and metadata to samples, tests, experiments etc, automating data transfer from instruments, and ensuring compliance with standards.
So how does a Scientific Data Management System (SDMS) compare to traditional file storage? And why should your lab consider making the switch? Let’s break it down.
Data Organization: From Folders to Intelligent Structure
One of the most critical differences between SDMS and traditional file storage is the automation of data capture and management of large-scale data. In a conventional file system, data is manually placed into folders, often depending on naming conventions and personal organization habits. Over time, this leads to a chaotic web of nested folders and duplicated files with names like ‘PH_Reading_1.xlsx’, ‘PH_Reading_2.xlsx’, or ‘PCR_V1.xlsx’, ‘PCR_V2.xlsx’. Searching for data can be frustrating, especially when multiple users are involved.
In contrast, an SDMS automatically organizes data based on metadata—such as project ID, instrument type, sample number, or user. This structured approach ensures consistency and findability, enabling researchers to retrieve and reuse data long after it’s been collected. It also supports scalability, so as your lab grows, your data remains clean, navigable, and connected.
Traceability and Version Control: Compliance Made Simple
Beyond just organization, traceability is another area where SDMS outshines file storage. In many labs, once a file is saved in a shared folder, there’s little to no record of what happens next. Who opened it? Who changed it? Was it the latest version? These questions become critical in regulated environments, where every action must be accounted for.
An SDMS maintains a complete audit trail, tracking every upload, modification, and access event. It supports version control, allowing users to compare changes and revert to previous versions when necessary—features that are crucial for meeting compliance standards such as 21 CFR Part 11 or ISO/IEC 17025.With an SDMS, you can trace data back to its source, understand its history, and verify its authenticity.
Automation and Integration: Reducing Manual Burden
Traditional file storage requires researchers to manually transfer data from instruments to folders, often wasting time and introducing the risk of errors. There’s also no easy way to parse, annotate, or link data across different systems. With an SDMS, these workflows are automated.
An SDMS can automatically capture data from instruments, apply parsing rules, and link data to relevant experiments or samples. It integrates with other digital lab systems such as LIMS (Laboratory Information Management Systems), ELNs (Electronic Lab Notebooks), and CDS (Chromatography Data Systems). This seamless connectivity builds a truly digital laboratory environment, enabling data to move efficiently and accurately between platforms—freeing scientists to focus on science instead of file handling.
Searchability and Metadata: From Guesswork to Precision
Search and retrieval are often overlooked in discussions about data management, but they’re crucial for any lab. In a traditional file system, search capabilities are limited—usually restricted to filenames or simple keyword matches. There’s little to no standardized metadata, which makes it hard to find files unless you know exactly what you’re looking for.
An SDMS, on the other hand, offers advanced search capabilities that allow users to find data using filters like project name, experiment type, date, instrument, or any custom metadata. This means locating a specific dataset or result takes seconds, not hours—speeding up decision-making and enabling faster research cycles.
Scalability and Collaboration: Enabling Teams, Not Hindering Them
As labs expand and projects span across departments or even continents, file storage systems often buckle under the pressure. Duplicate files, inconsistent versions, and rigid permission settings make collaboration clunky and error prone. These issues only intensify in larger, interdisciplinary teams where access needs and workflows vary widely.
An SDMS resolves these challenges by providing a centralized platform with scalable architecture and smart access controls. Role-based permissions ensure each team member—whether a researcher, analyst, or QA lead—sees exactly what they need, while real-time updates and version control keep everyone aligned. By eliminating data silos and offering a shared workspace, an SDMS fosters efficient, secure collaboration at scale—making it easier to work together, wherever your team is based.
Compliance and Risk Mitigation: Built-In Confidence
Regulatory compliance is not optional for many labs. Whether you’re in pharmaceuticals, clinical research, or food testing, governing bodies expect meticulous records, traceable data, and demonstrable control.
File storage systems don’t offer the audit logs, secure access controls, or retention tools necessary to meet these demands. An SDMS is designed with compliance in mind. Features like electronic signatures, role-based access, automated data retention, and complete audit trails give labs confidence during inspections and audits. This reduces not only the risk of fines and violations but also the internal stress and resource strain of regulatory readiness.
Real-World Impact: A Case in Point
A mid-sized pharmaceutical lab has shifted from cloud-based file storage to a Scientific Data Management System (SDMS). Before the switch, researchers spent hours each week locating files or reconstructing lost datasets. Collaboration across departments was sluggish, and audit preparation was a burdensome, manual process.
After implementing an SDMS, the lab reported an 80% reduction in data retrieval time, a 60% decrease in duplicate files, and a 50% improvement in audit readiness. These improvements weren’t just operational—they enabled faster product development, smoother regulatory submissions, and improved cross-functional coordination.
It’s Time to Move Beyond Files & Folders
Traditional file storage systems can no longer meet the demands of modern scientific research. As data complexity increases and collaboration evolves, labs need more than just a place to store files; they require a smart, scalable Scientific Data Management System (SDMS).
Switching to an SDMS can boost productivity, reduce compliance risks, and unlock the full potential of research. If you’re ready to enhance your data management, Logilab SDMS offers a robust platform designed specifically for scientific environments, facilitating a smoother transition.
Request a demo or Brochures- Agaramtech to see how Logilab SDMS can transform your lab’s data management strategy.
