In the rapidly advancing realm of laboratory management, understanding the “LIMS process flow” is paramount. The Laboratory Information Management System (LIMS) has emerged as the backbone of modern labs. Predictions for 2023 indicate that the global LIMS market will reach an impressive $2.5 billion, boasting a CAGR of 8.9% since 2021. With 77% of research-intensive firms integrating LIMS, the importance of “LIMS modules” in contemporary labs cannot be overstated.
Difference between Legacy and Modern LIMS solutions
| Aspect | Legacy LIMS Software | Modern LIMS Software |
| Technology | Relies on client server-based solutions | Utilizescontemporary technology, offering adaptability. |
| Integration | Limited integration with instruments and systems, leading to manual data entry. | Robust integration capabilities for seamless communication with lab equipment and systems, reducing errors. |
| User Interface | Often features less intuitive and complex user interfaces. | Designed with user-friendly interfaces, reducing the learning curve. |
| Customization | Limited customization options, challenging to tailor to specific lab workflows. | Extensive customization options, enabling tailoring to unique lab requirements. |
| Compliance | It may lack features to meet modern regulatory requirements, such as 21 CFR Part 11. | Includes features designed for regulatory compliance, ensuring data integrity and security. |
| Data Accessibility | Data retrieval and analysis may be cumbersome, slowing down lab operations. | Streamlined data retrieval and analysis, providing faster access to critical information. |
| Scalability | May struggle to accommodate growing data volumes and ever-changing lab needs. | Designed to scale with the growing needs of modern labs, accommodating data growth and user expansion. |
| Reporting and Analytics | Reporting and analytics tools may be rudimentary or nonexistent. | Offers robust reporting and analytics tools, empowering data-driven insights. |
| Mobile Accessibility | Typically, it lacks mobile accessibility. | It often provides mobile access for remote data access and tasks. |
| Vendor Support | May have limited or outdated customer support options. | Reputable vendors offer comprehensive customer support, including training and technical assistance. |
As we venture into 2023, the Future of Laboratory Information Management System (LIMS) landscape is poised for even more transformative changes. The rapid advancements in technology, coupled with the ever-evolving needs of modern laboratories, are shaping the future of LIMS in unprecedented ways. Here is a glimpse into what LIMS 2023 looks like.
Why Qualis LIMS is the Gold Standard:
In the relentless pursuit of scientific innovation, labs equipped with state-of-the-art LIMS, such as Qualis, stand at the forefront. As we navigate the digital era, it is evident that a LIMS-powered lab is not just an advantage but an imperative. Standing tall among LIMS vendors, Qualis LIMS is an innovative Laboratory Information Management System tailored for diverse industries. It embodies the future, driving labs toward automation, ensuring stringent regulatory compliance, and upholding industry standards.
Dynamic Sample Management for Versatile Labs:
Qualis LIMS excels in accommodating the diverse needs of laboratories, allowing them to handle various categories of samples with ease. Whether it is raw materials, finished products, in-process samples, or patient specimens, Qualis LIMS provides the flexibility to create specific sample registration templates for each category. This adaptability empowers laboratories to streamline their processes and meet their unique requirements efficiently. High-performance labs thrive on this capability, effortlessly meeting critical deadlines.
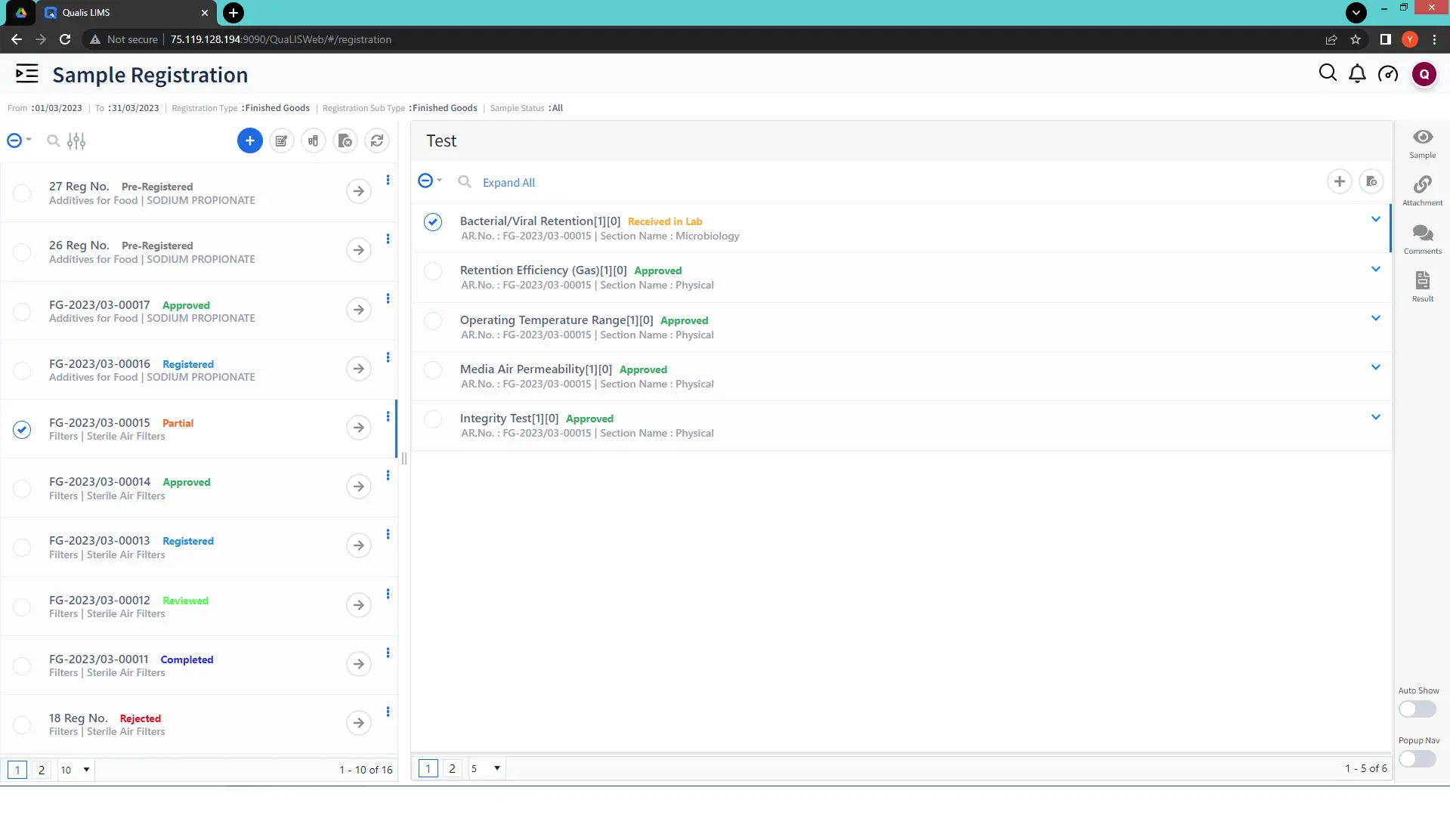
Optimized Resource Planning for Peak Performance:
Qualis LIMS does not just manage samples; it is a powerful tool for resource planning. By intelligently allocating certified equipment and personnel, laboratories ensure peak utilization, resulting in enhanced overall lab performance. This strategic LIMS resource management is pivotal in maintaining operational efficiency and reducing downtime.
Meticulous Management of Good Laboratory Practices (GLP):
For GLP laboratories, adhering to regulations and standards is non-negotiable. Qualis LIMS simplifies this complex task by facilitating the execution of mandatory internal procedures. This meticulous approach not only ensures regulatory compliance but also contributes to the consistently high quality of testing results.
Streamlined Automation for Productivity Gains:
Qualis LIMS embraces automation to drive considerable time savings and boost productivity. Automated processes reduce manual intervention, minimizing the chances of errors, and accelerating workflows. This efficiency translates into quicker turnaround times and optimized resource allocation.
Intuitive User-Centric Design:
Qualis LIMS places user experience at the forefront with its intuitive design. The system is designed to be user-friendly, instilling confidence in its users. This user- centric approach promotes smoother adoption and utilization of the LIMS, enhancing operational efficiency.
Rigorous Regulatory Adherence:
Qualis LIMS is purpose-built to meet the most stringent regulatory requirements. It offers laboratories peace of mind by ensuring continuous compliance with evolving industry standards and regulations. This commitment to regulatory adherence is a hallmark of Qualis LIMS.
Summary:
In 2023, Laboratory Information Management Systems (LIMS) have become essential for modern labs, with a projected global market value of $2.5 billion. Legacy LIMS systems differ from modern ones, which leverage advanced technology, robust integration, user-friendly interfaces, customization options, regulatory compliance features, and scalability.
Qualis LIMS is a standout in the field, offering innovation, automation, regulatory compliance, and industry standards support. It excels in dynamic sample management, optimized resource planning, meticulous Good Laboratory Practices (GLP) management, streamlined automation, user-friendly design, and rigorous regulatory adherence, making it a vital tool for modern laboratories.





