An Industry-Agnostic LIMS Solution
Qualis LIMS is a configurable Laboratory Information Management System (LIMS software) that unifies your lab operations into a secure, paperless, and compliant cloud-based environment—from sample receipt to final report delivery and beyond. Designed for industries where precision and regulation are critical, Qualis LIMS offers an intuitive way to manage samples, execute tests, automate workflows, and record results from a central secure platform.
Qualis LIMS provides integrated out-of-the-box modules that can be used by any QC, Research, Healthcare or Commercial lab. It provides a structured method to document laboratory investigations & results while adhering to various regulatory compliance requirements (21 CFR Part 11, EudraLex Annex 11, ISO 17025, GLP, GAMP 4)
higher productivity via automated workflows & reporting
faster audit readiness due to full data traceability
fewer transcription errors in data entry
faster sample testing throughput
Flexible Sample Registration & Testing
Register and test any sample type - raw materials, finished products, patient samples, clinical trial specimens, or R&D project samples
- Dynamic sample registration templates with drag-and-drop fields .
- Version-controlled template management for data integrity
- Sample lifecycle tracking with complete metadata capture
Beyond Basic Lab Management
Empowering Efficiency, Compliance, and Growth.
1
Sample Pre-Registration/ Registration
2
Result Entry (Manual, Instrument interface)
3
Approval & Release (HOD, Study Director, CRO)
4
Reports
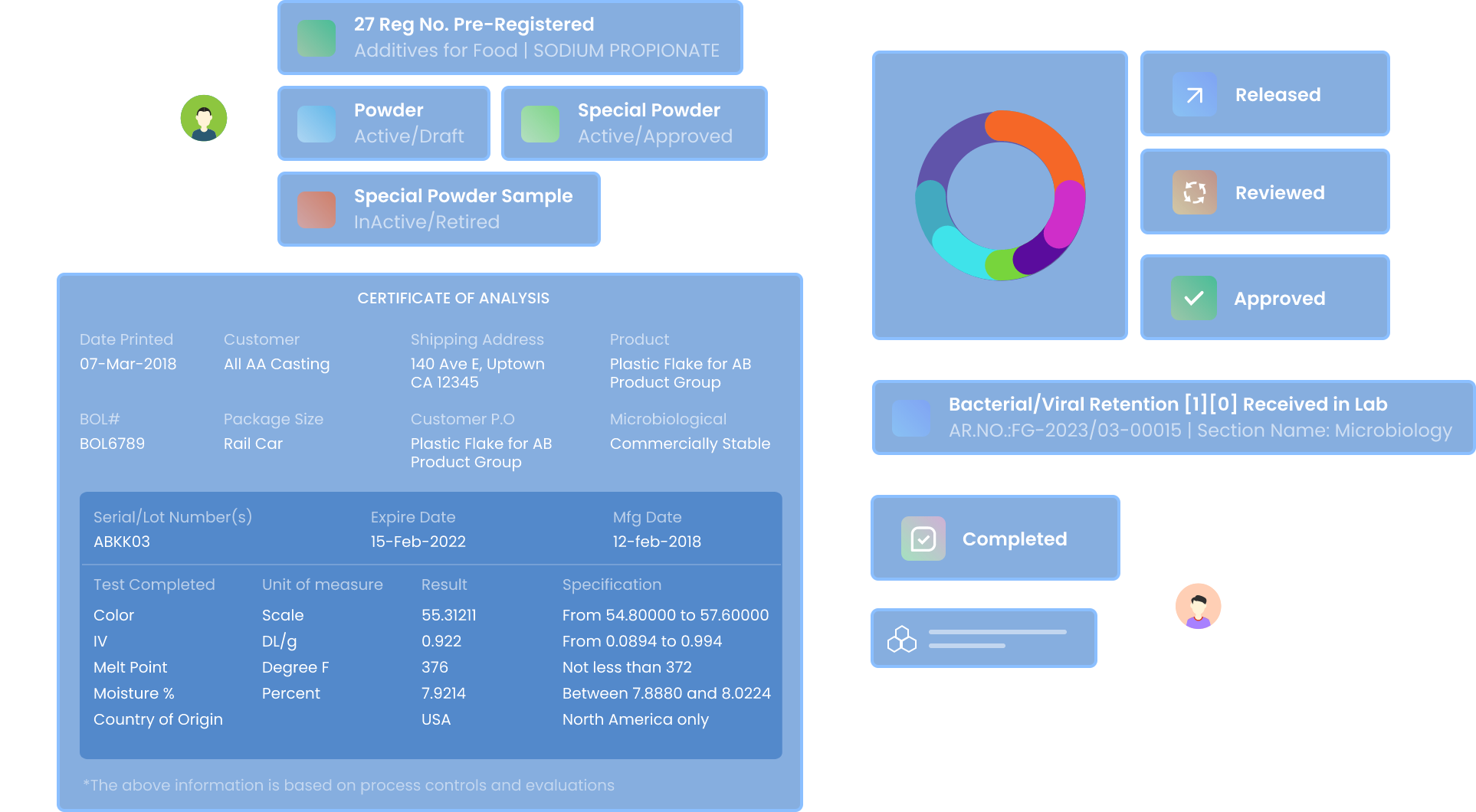
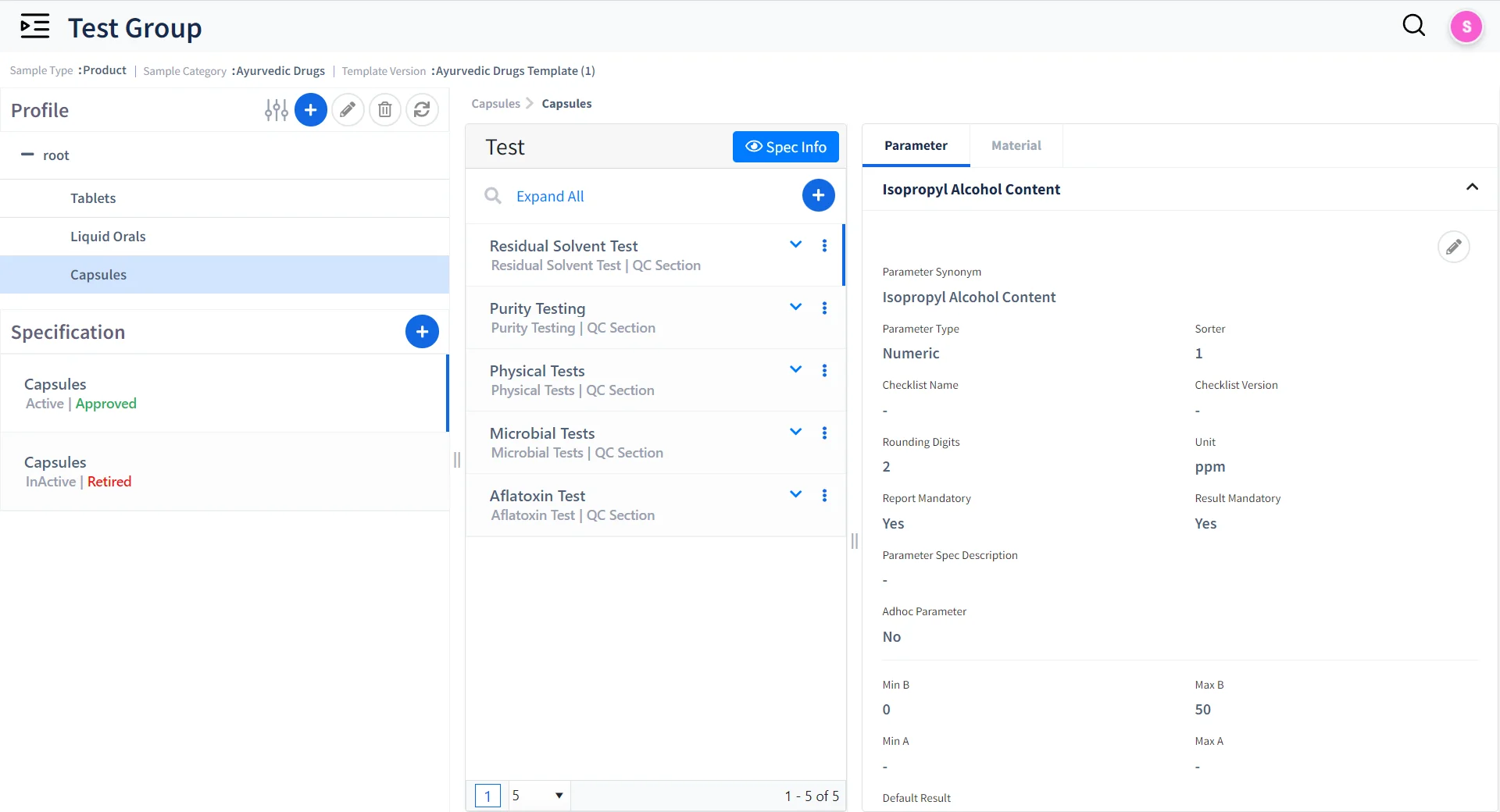
Unified Test and Specification Management
-
Manage Test Groups
Create and manage multiple test groups for samples based on Test Parameters.
-
Maintain Traceability
Manage test specifications with Version control and release control
-
Define Test Specifications
Set Limits based on expected test Results
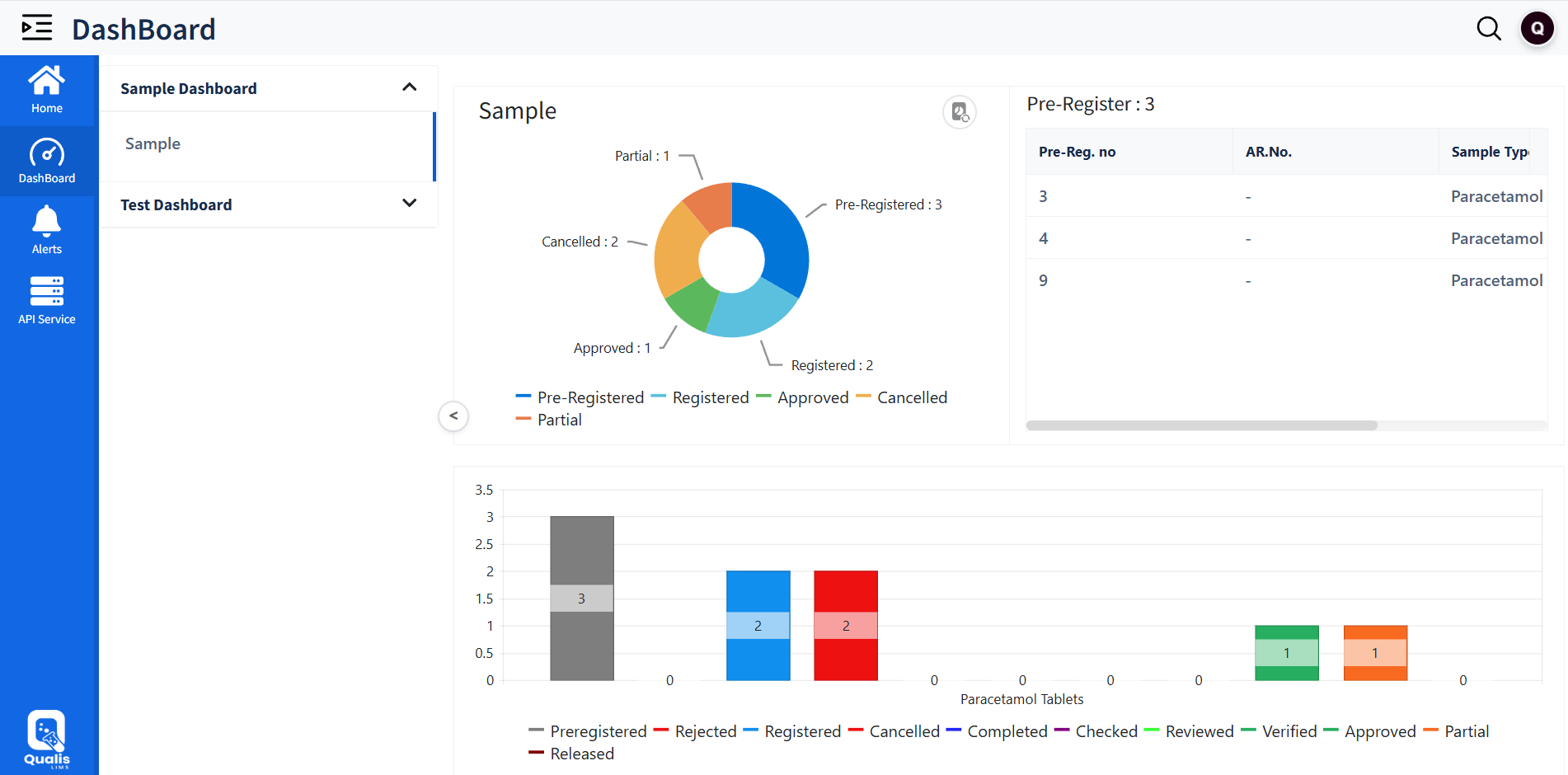
Live Dashboards & Alerts
-
Comprehensive Dashboard
Real-time KPI dashboards for operational visibility
-
Intuitive Alerts
Automated alerts for approvals, calibration due dates, job assignments, and non-conformance eventslity
-
Controlled View
Customizable, role-based views for managers, analysts, and QA
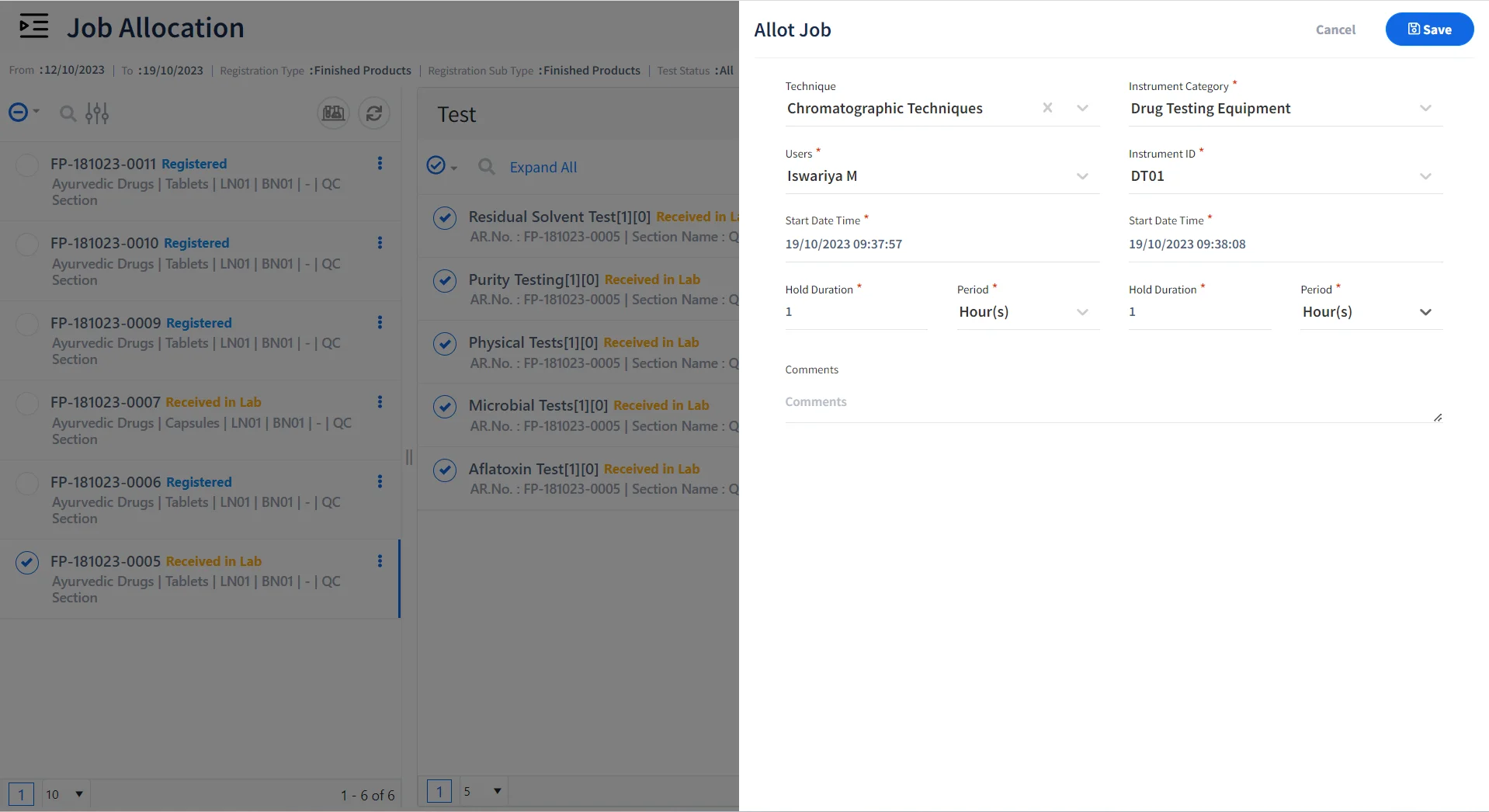
Allocate Jobs to Certified Personnel & Qualified Instruments
-
Allot Jobs
Allocate tests, instruments, and samples to be tested for various personnel.
-
Assign Role-Specific Tasks
Allocate Role based tasks to lab personnel for performing specific tests.
-
Allocate Tests to Certified Personnel
Ensure that certified personnel are allocated to specific jobs or techniques.
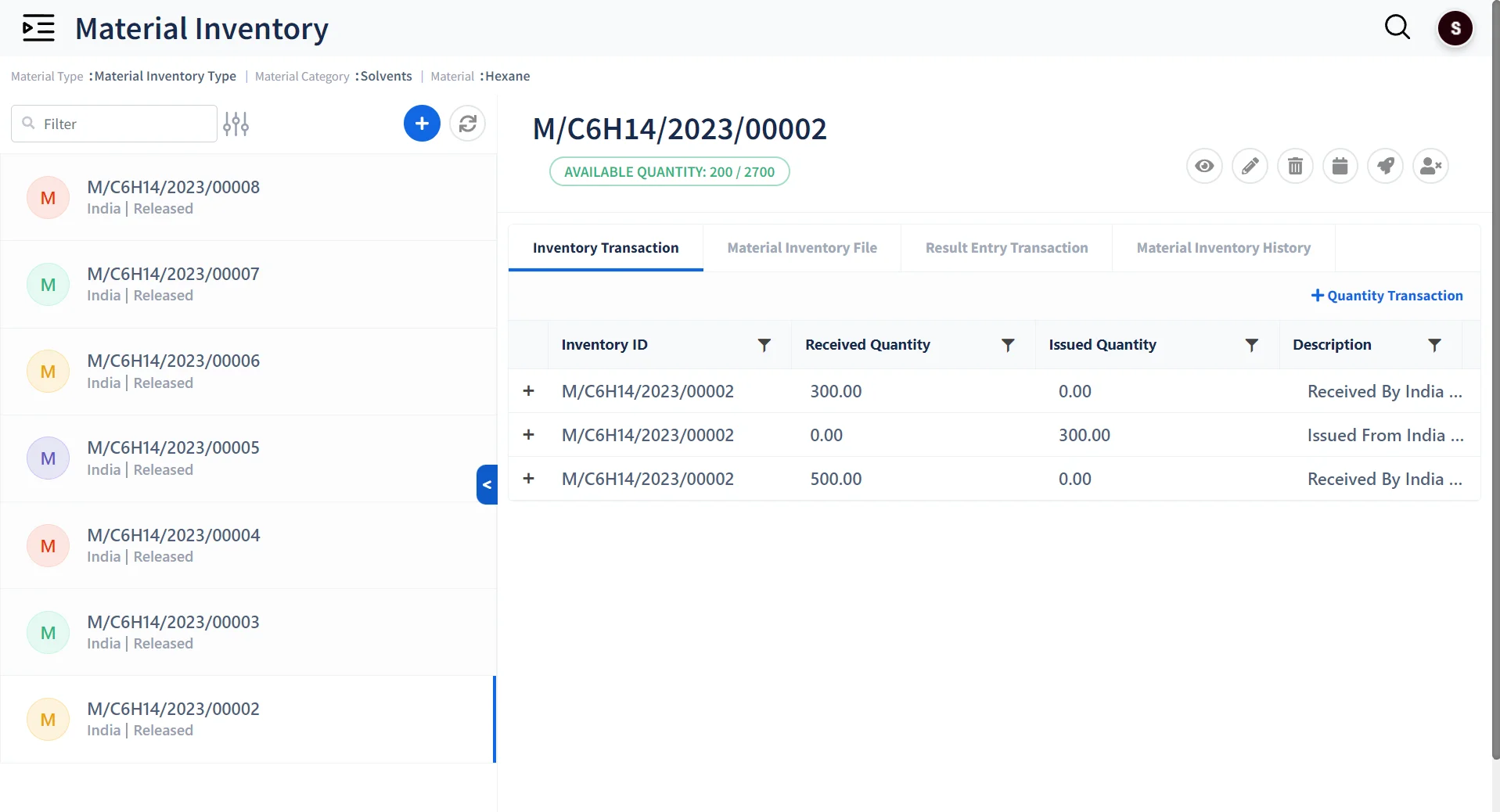
Standardized Inventory Management
-
Assign Barcode Labels
Maintain Multiple Categories of Inventory Material with Barcode labeling
-
Track Inventory Levels
Record and track usage of Inventory .
-
Optimize Material Storage
Create Dynamic Storage Structures for Storing Materials
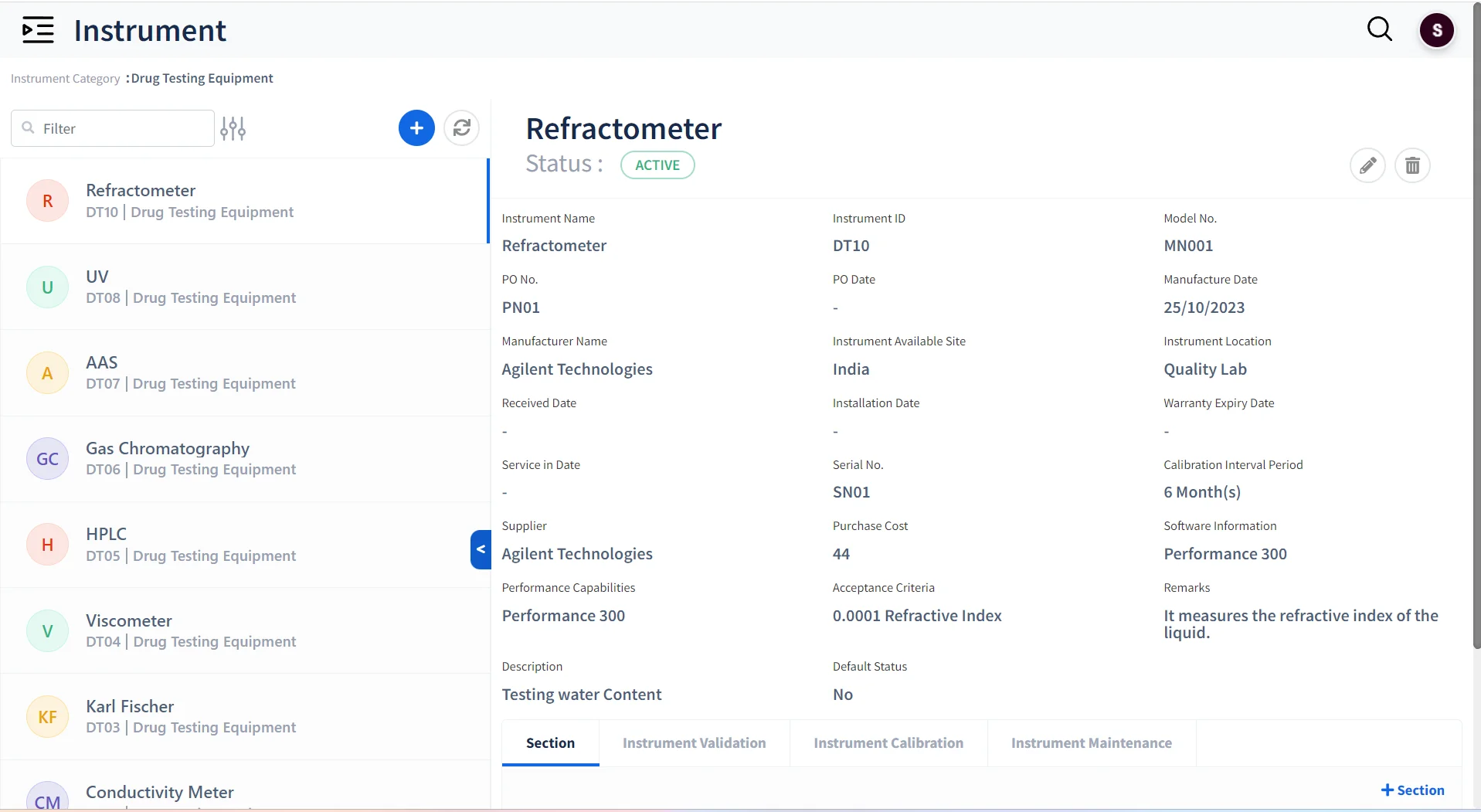
Instrument & Equipment Management
-
Track Instruments Usage
Maintain all instruments and equipment used across the laboratory
-
Maintain Instrument Logs
Keep track of the Validation, Calibration and maintenance of instruments
-
Verify Instrument Reliability
Ensure that only calibrated instruments can be assigned for tests.
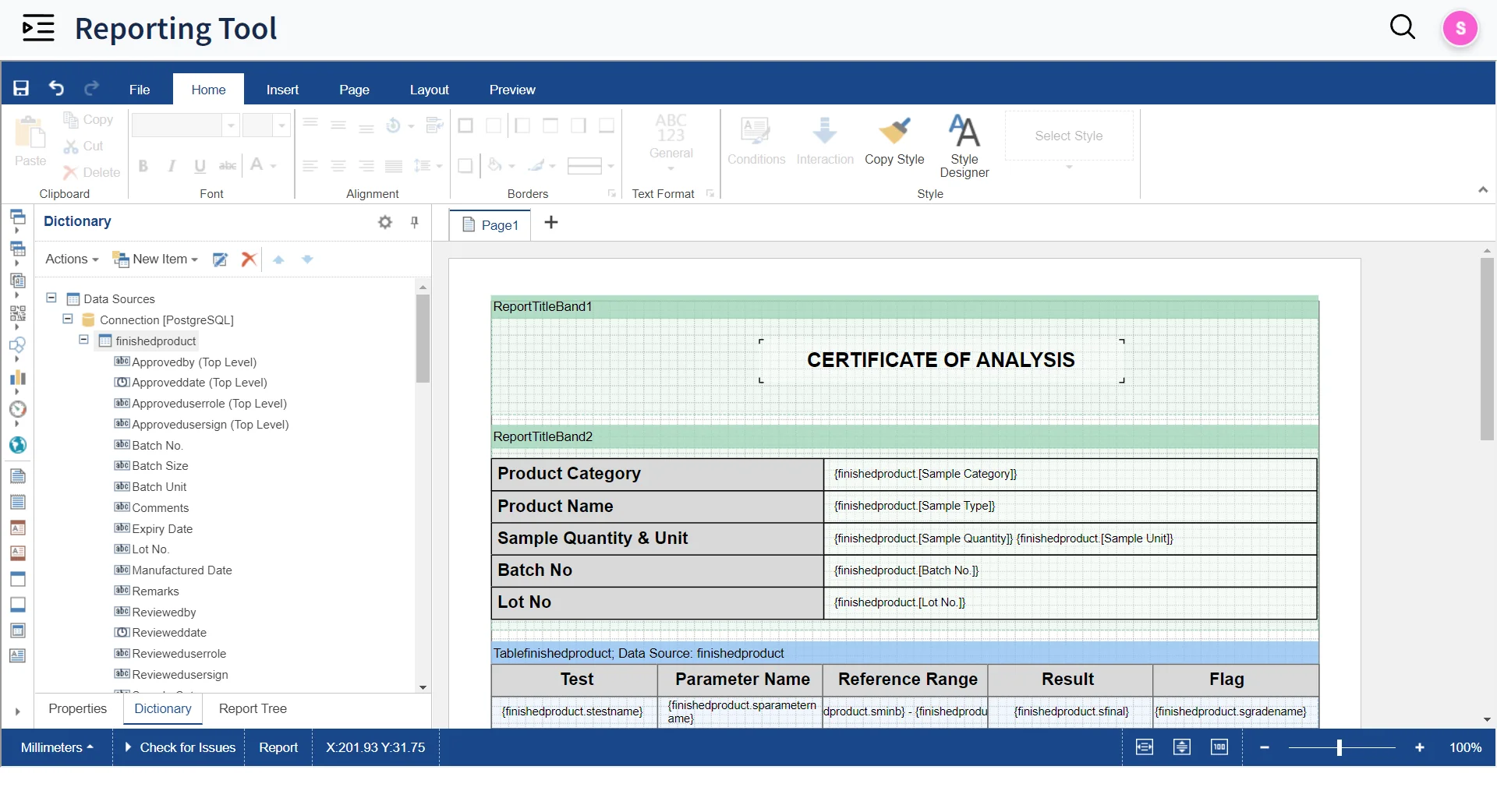
Automated Result Entry & Reporting
-
Design Report Templates
Design Templates for reports with Built-In Reporting Tool and auto-generate SQL Queries for reporting with Query Builder
-
Approve & Release
Approve and Release reports in seconds with Electronic and digital signatures
-
External Report Access
Make reports available for external stakeholders through Qualis Portal
Why Modern Labs choose Qualis LIMS

Web-based & platform-independent
Chrome, Firefox, Safari etc

Highly scalable
multi-site, multi-department, multi-lab

No-code configurability
tailor workflows without programming

Compliance-ready
21 CFR Part 11, EudraLex Annex 11, ISO 17025, GLP, GAMP 4

Lower cost of compliance
pre-validated modules and audit-ready features
Industries & Use Cases

Pharmaceutical Quality Control & QA
Batch release, finished product testing, stability studies, CAPA tracking, deviation documentation, and compliance with data integrity standards such as 21 CFR Part 11 and EudraLex Annex 11

Healthcare & Clinical Diagnostics
Patient sample tracking with full chain-of-custody, integration across hospital and diagnostic networks, and improved testing throughput through automated data exchange and quality control traceability.

Life Sciences Research
Centralized management of research data, sample metadata, and assay tracking enabling multi-site collaboration, version-controlled documentation, and reproducible experimental workflows

Food Safety & Quality Testing
Ingredient traceability, microbial and chemical QC testing, and compliance documentation aligned with regulatory and quality standards

Environmental & Water Testing Labs
Field-to-lab sample management, monitoring of contaminants and water parameters, and adherence to local and international environmental regulatory standards

Chemical & Industrial Manufacturing
GLP-compliant QC testing, deviation and CAPA management, process validation, and data-driven optimization for continuous quality improvement


Compliance & Audit-Readiness
Designed with ALCOA+ principles, audit trails, role-based access control, and electronic record integrity. und elektronischer Datensatzintegrität. Qualis LIMS helps teams stay inspection-ready for FDA, EMA, NABL, and ISO audits, minimizing preparation time and documentation effort.
Key Benefits at a Glance

Complete Workflow Automation
from sample registration to report release

Regulatory Compliance Built-In
21 CFR Part 11, ISO 17025, GLP, GAMP 4

Enhanced Data Integrity
ALCOA+, secure audit trails, granular access

Faster Turnaround Time (TAT)
alerts, dashboards, high-throughput sample testing

Scalable for Any Lab Size
single site to global multi-location

Cost-Effective Deployment
SaaS or on-premise with low IT overhead
Deployment Flexibility
Deploy Qualis LIMS on AWS, Azure, customer-owned private cloud, or on-premises infrastructures
Deployment Flexibility
Deploy Qualis LIMS on AWS, Azure, customer-owned private cloud, or on-premises infrastructures




Customer Success Snapshot
Global Healthcare Diagnostics Provider — Multi-Site Rollout

Challenge:
Manual sample tracking and fragmented reporting caused delays and compliance risk
Solution:
Deployed Qualis LIMS across 8 facilities with instrument connectivity, centralized dashboards, and barcode-enabled inventory.
Results:
Results: 40% faster TAT, 100% audit readiness within 3 months, and seamless cross-site collaboration.
Frequently Asked Questions
Download Compliance Datasheet
Would you like to learn more about Qualis LIMS?
Submit this form and our sales representative will
contact you soon.
Email Us:
[email protected]