Agaram Technologies Visits Analytica 2024 — April 9-12
Attend our 3-day product exhibitJoin Agaram Technologies at Munich where you can learn about how our laboratory automation solutions can enable paperless processes and compliance.






Achieve Complete Process Automation, Digitalisation and Compliance across Laboratory Operations
Seamlessly manage your tests, samples, experiments & reports, Capture data from any lab instrument,
Record and execute your test procedures and more !

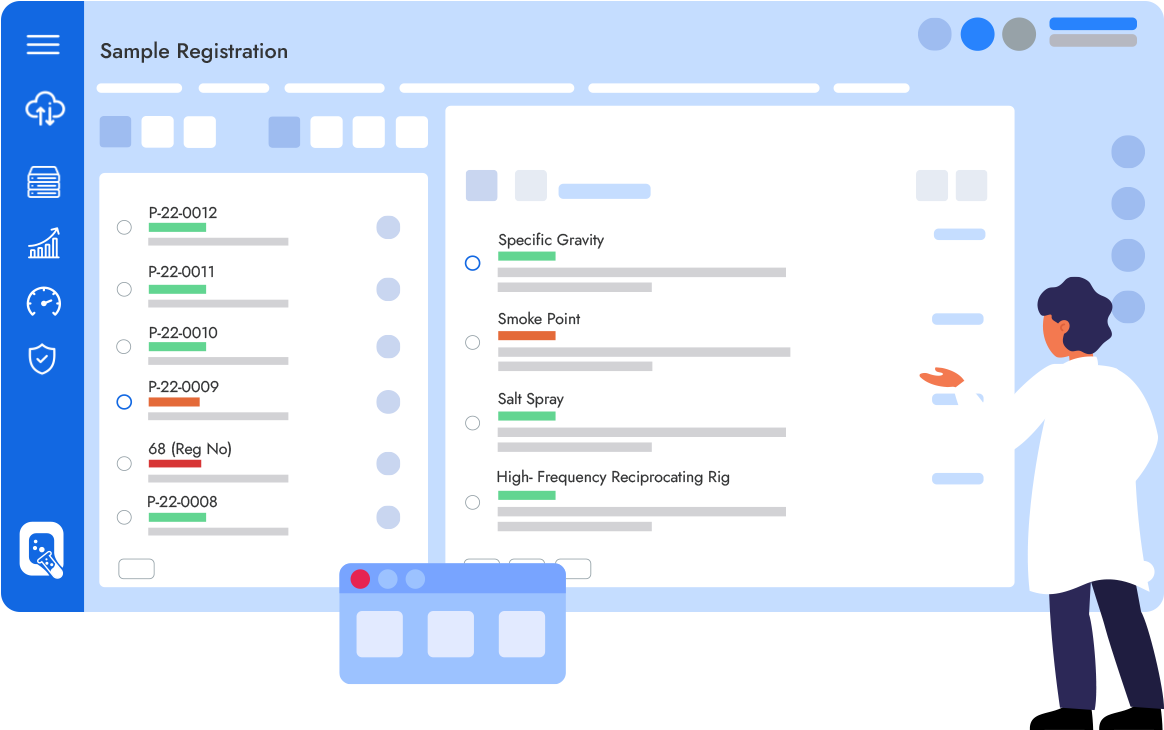
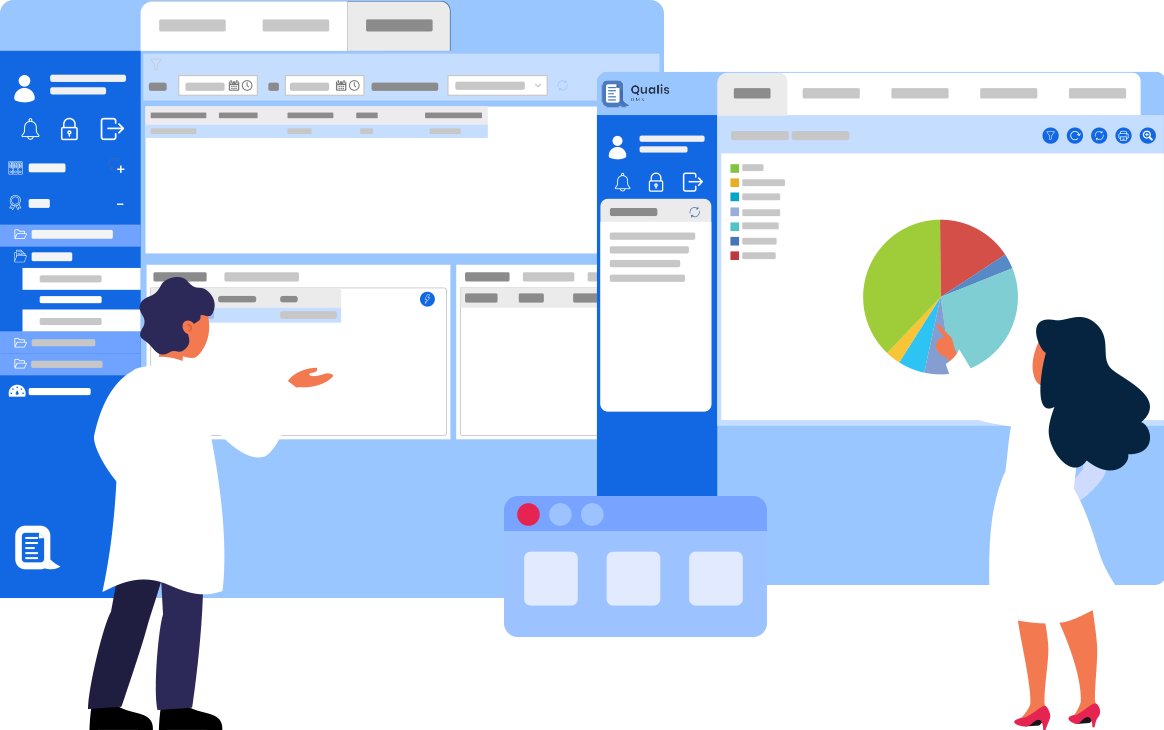
Qualis LIMS is an enterprise class laboratory information management system designed to digitally transform Laboratories by enabling a fully paperless environment for sample testing, automating laboratory processes & Regulatory Compliance.

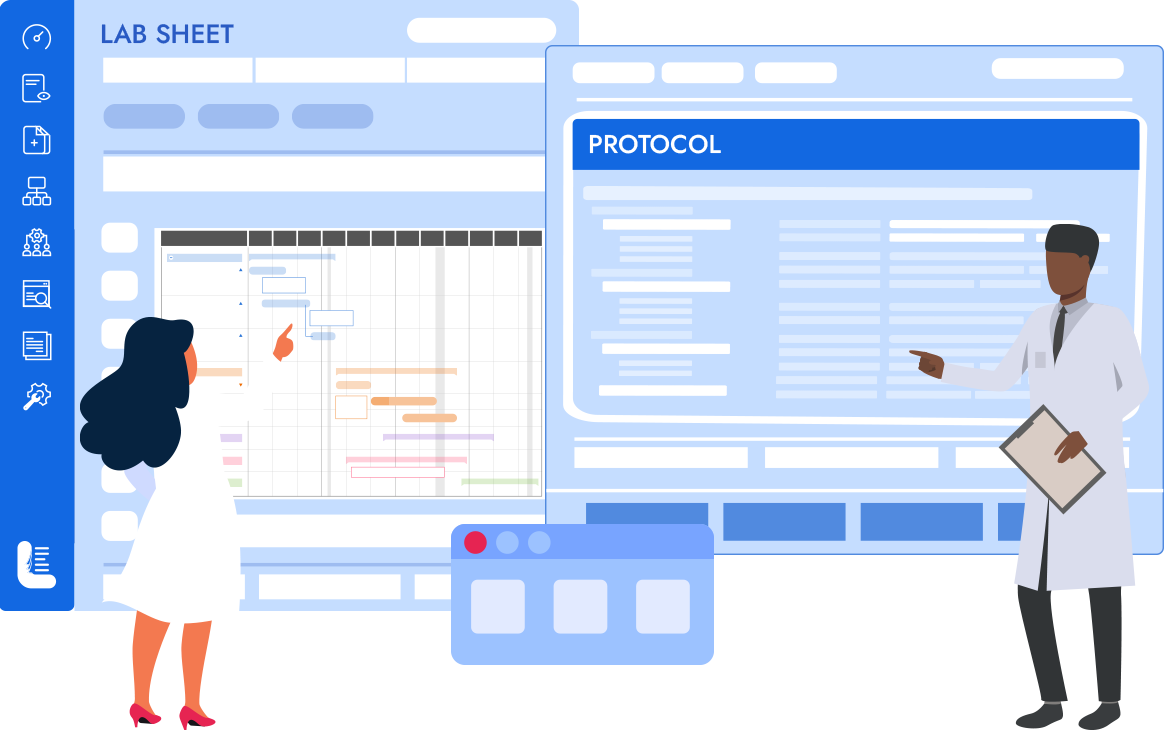
Logilab ELN is a cutting-edge Electronic Lab Notebook solution tailored for Quality Control & Research Labs. It empowers labs to record, execute, and store test & experimental data in a compliant paperless setting.

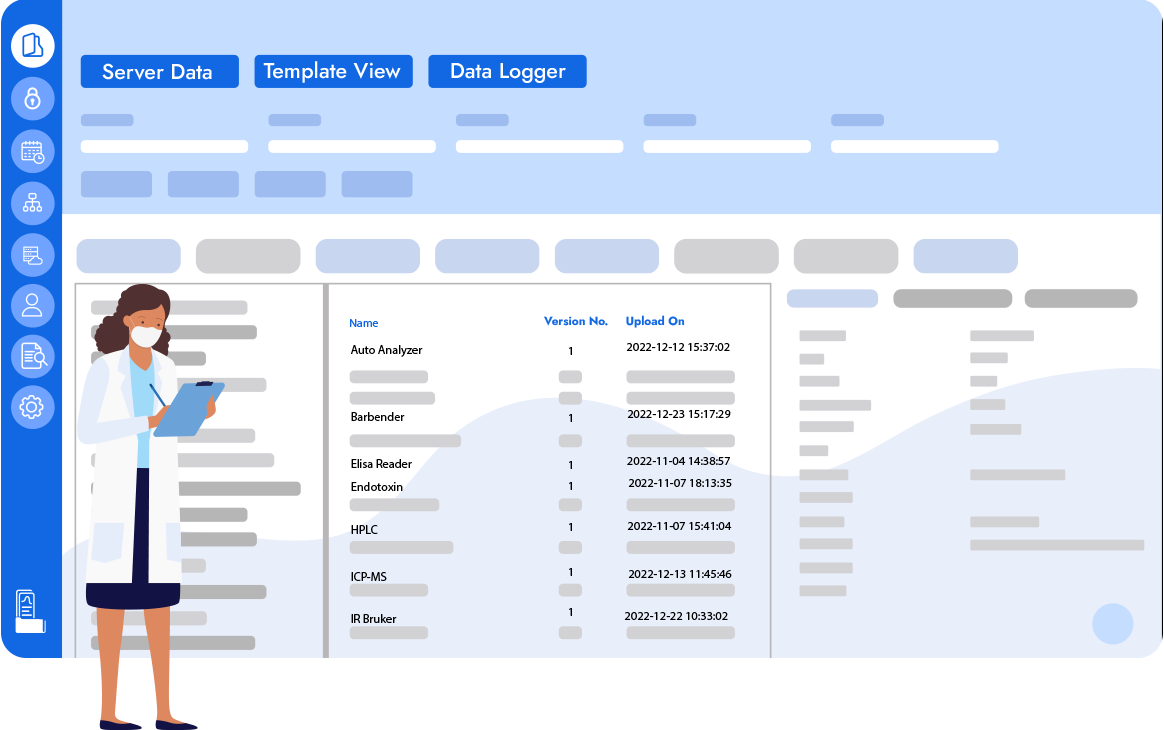
Logilab SDMS is an advanced scientific data management platform that enables a central reliable source of truth for Capture, Catalogue & Archival of Instrument Raw Data & Metadata all while maintaining 21 CFR Part 11 Compliance.

Qualis DMS is a unified Document Management Platform for regulated organizations. It seamlessly Connects, Manage and Streamlines documents, ensuring full control, traceability, and compliance.
Adhere to Industry standards, Follow Good Laboratory Practices & comply with 21 CFR Part 11 requirements
Get Started with our products,
Ask for a Demo
Seeing is believing , Reach Out to us for a Discussion or Product Demonstration
Global Presence across Regulated and Non-Regulated Industries
Headquartered in Chennai, Agaramtech delivers its software solutions to over 30+ countries worldwide, catering to diverse industries such as Pharmaceuticals, Life-Sciences and Biotech, Healthcare and Diagnostics, Dairy, Food & Beverage, Oil & Gas, Chemical, Process Manufacturing, Environment, etc
Over Six Decades of Laboratory Knowledge
Established in 1959, Agaram has been a part of the laboratory space for the last 6 decades. Our team has over 25 years of experience in the design & development of laboratory informatics solutions.
Customers
Countries
Years Of Experience
Customer Success Stories
Discover how our solutions have empowered labs across industries. From aiding a COVID-19 vaccine manufacturer in achieving Data Integrity, Compliance and Automation to streamlining Pharma manufacturing processes, our impact resonates across industries.
Resources and Learning
Stay updated with our latest brochures, blogs, and white papers. Dive deep into the potential of our solutions and how they can benefit your team.